Antitumor drug PEGylation and applications of antitumor drug PEGylation in reversal of tumor multidrug resistance
An anti-tumor drug and tumor technology, applied in the direction of anti-tumor drugs, drug combinations, medical preparations of non-active ingredients, etc., can solve the problems of chemotherapy failure, cumulative cardiotoxicity, strong side effects, etc., and achieve immunogenicity and Decreased antigenicity, improved pharmacokinetic properties, and prolonged half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
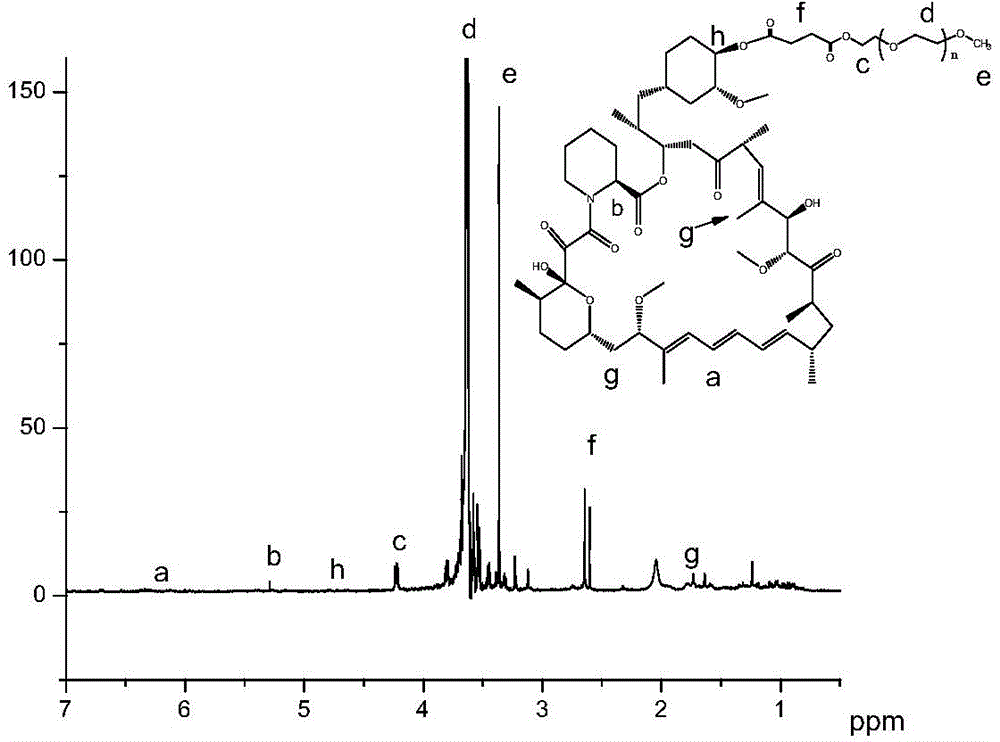
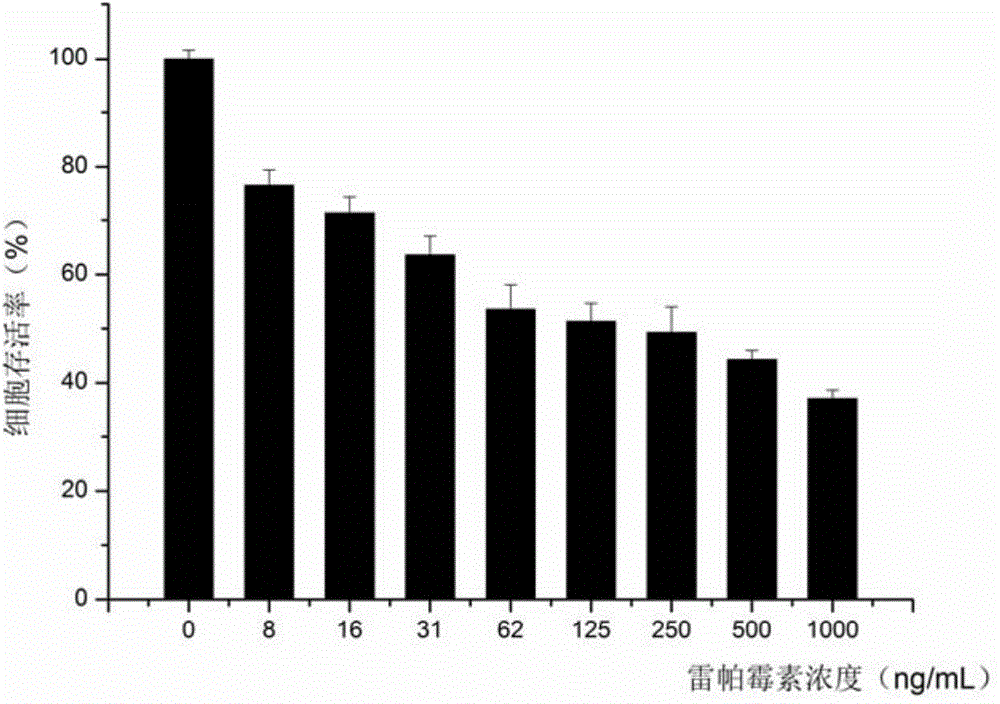
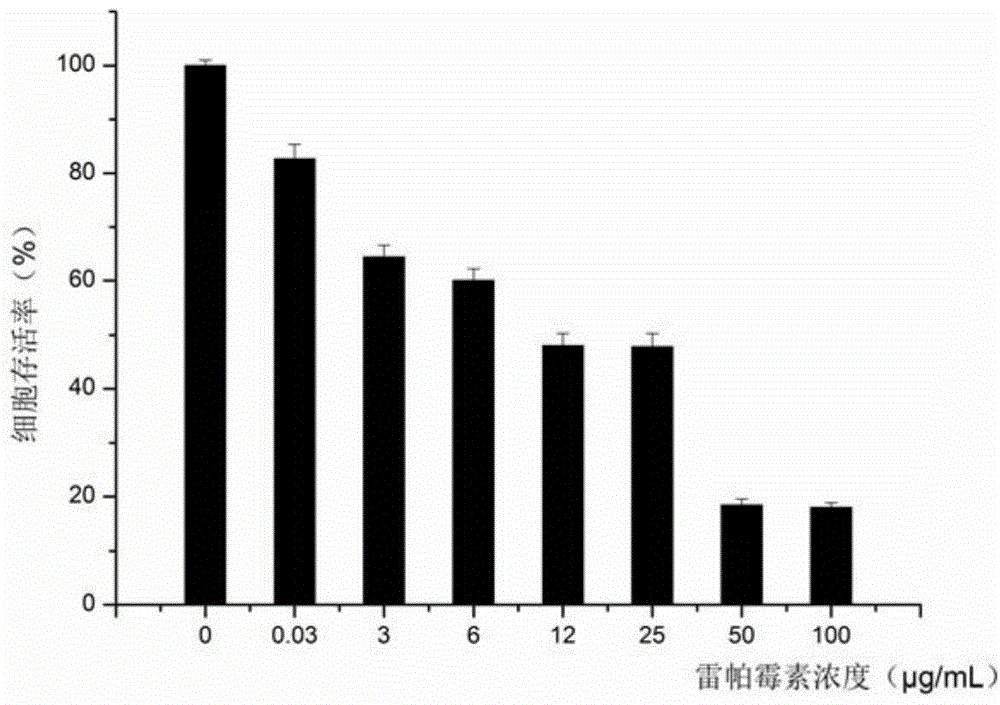
[0042] Taking PEG-RAPA as an example to illustrate its synthesis process, determination of physical and chemical properties and its reversal effect on MCF-7 / ADR cells:
[0043] Synthesis of PEG-RAPA: Dissolve 88 mg of mPEG-COOH in 10 mL of ice-free dichloromethane, then add 107 mg of rapamycin, 30 mg of DCC and 15 mg of DMAP. The temperature was naturally raised to room temperature, and the reaction was stirred for 3 d. After the reaction was completed, it was filtered, anhydrous ether precipitated twice, and then the precipitate was vacuum-dried to obtain the obtained product. PEG-RAPA (PEGylated Rapamycin) 1 H NMR spectrum as figure 1 shown.
[0044] Preparation of PEG-RAPA micelles and determination of particle size: PEG-RAPA micelles were prepared by solid dispersion method. Dissolve 1 mg PEG-RAPA in 100 μL of acetonitrile, evaporate to dryness naturally, dissolve in 2 ml of ultrapure water, vortex for 3 min, sonicate for 5 min, centrifuge at 12000 r / min for 10 min, ta...
Embodiment 2
[0064] Taking PEG-PTX as an example to illustrate its synthesis process, determination of physical and chemical properties and its reversal effect on MCF-7 / ADR cells:
[0065] Synthesis of PEG-PTX: Dissolve 88 mg of mPEG-COOH in 10 mL of anhydrous dichloromethane, cool to 0°C on ice, then add 100 mg of paclitaxel, 30 mg of DCC, and 15 mg of DMAP, stir and react, naturally warm to room temperature, and react for 3 days . After the reaction was completed, it was filtered and precipitated with glacial ether, and then the precipitate was vacuum-dried. PEG-PTX (PEGylated Paclitaxel) 1 H NMR spectrum as Image 6 shown.
[0066] Preparation of PEG-PTX micelles and determination of particle size: PEG-PTX micelles were prepared by solid dispersion method. Dissolve 1 mg PEG-PTX in 100 μL of acetonitrile, evaporate to dryness naturally, dissolve in 2 ml of ultrapure water, vortex for 3 min, sonicate for 5 min, centrifuge at 12000 r / min for 10 min, take the supernatant and filter it w...
Embodiment 3
[0082] Taking PEG-DOX as an example to illustrate its synthesis process, determination of physical and chemical properties and its reversal effect on MCF-7 / ADR cells:
[0083] Synthesis of PEG-DOX: Take 210mg mPEG-COOH, 58mg doxorubicin hydrochloride, 23mg NHS, 20mgDCC, 12mgTEA in 10ml of anhydrous dichloromethane, react at room temperature for 3 days under dark nitrogen, filter, dialyze, and freeze-dry. PEG-DOX (PEG Adriamycin) 1 H NMR spectrum as Figure 11 shown.
[0084] Preparation of PEG-DOX micelles and determination of particle size: PEG-DOX micelles were prepared by solid dispersion method. Dissolve 1 mg PEG-DOX in 100 μL of acetonitrile, evaporate to dryness naturally, dissolve in 2 ml of ultrapure water, vortex for 3 minutes, sonicate for 5 minutes, centrifuge at 12000 r / min for 10 minutes, take the supernatant and filter it with a 0.45 μm microporous membrane. have to.
[0085] The particle size of PEG-DOX micelles is measured by dynamic light scattering (DLS):...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com