Production method of polysubstituted biphenyl compound and solid catalyst used in the production method
A production method and technology for benzene compounds, which are applied in the preparation of carbon compound catalysts, organic compounds, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of complex reaction system and short catalyst life, and achieve high yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
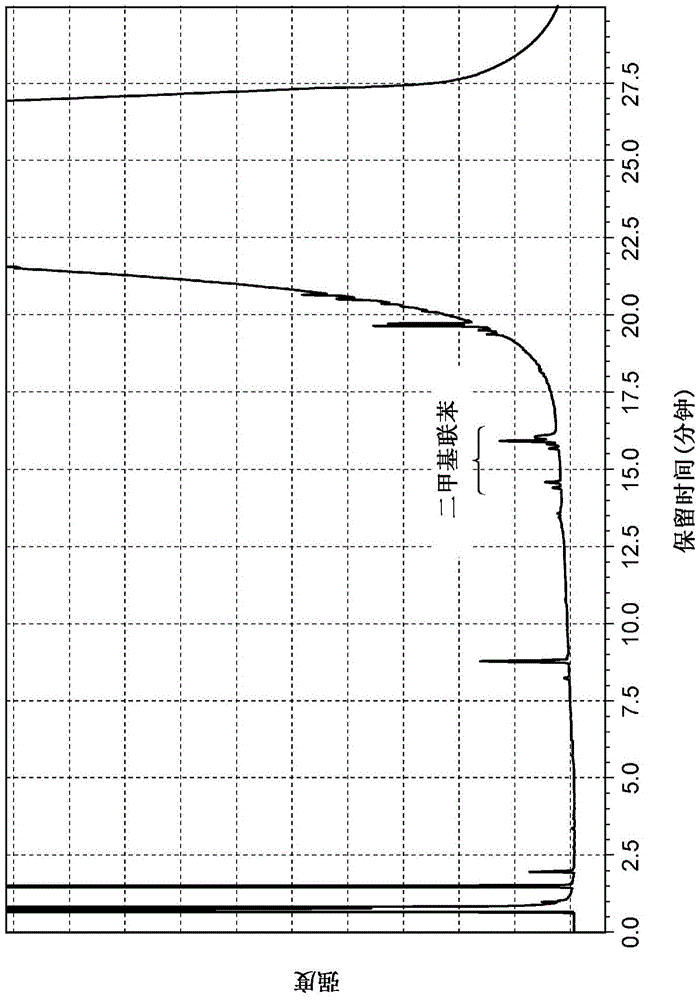
[0136] Example 1-1 (A solid catalyst with gold immobilized on cobalt oxide (hereinafter also referred to as "Au / Co 3 o 4 ")Synthesis)
[0137] 5.52 g of cobalt(II) nitrate hexahydrate and 0.41 g of tetrachloroauric acid tetrahydrate were dissolved in 200 mL of distilled water at room temperature (aqueous solution 1). On the other hand, 2.63 g of sodium carbonate was separately dissolved in 200 mL of distilled water (aqueous solution 2). Next, the above-mentioned aqueous solution 1 was added to the above-mentioned aqueous solution 2 at one time, and the mixture was stirred at room temperature for 3 hours. The resulting precipitate was washed with distilled water until the pH became constant, and filtered. The filtrate was dried overnight at 70°C. After drying, it was calcined at 300° C. in air for 4 hours to obtain a solid catalyst (Au / Co 3 o 4 ). The average primary particle diameter of the obtained solid catalyst was about 15 nm, the immobilized amount of gold was 10...
Embodiment 1-2
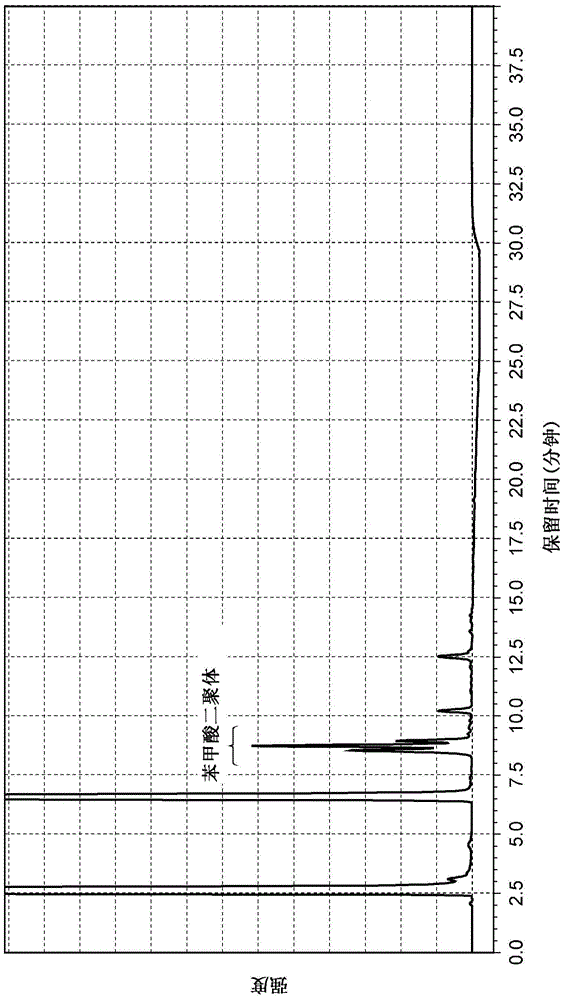
[0138] Example 1-2 (Synthesis of a solid catalyst with gold immobilized on nickel oxide (hereinafter also referred to as "Au / NiO"))
[0139] 5.53 g of nickel nitrate hexahydrate and 0.41 g of tetrachloroauric acid tetrahydrate were dissolved in 200 mL of distilled water at room temperature, and heated to 70° C. (aqueous solution 3). On the other hand, 2.67 g of sodium carbonate was separately dissolved in 250 mL of distilled water, and heated to 70° C. (aqueous solution 4). Next, the above-mentioned aqueous solution 3 was added to the above-mentioned aqueous solution 4 at one time, and the mixed solution was stirred at 70° C. for 1 hour. The resulting precipitate was washed with distilled water until the pH became constant, and filtered. The filtrate was dried overnight at 70°C. After drying, the solid catalyst (Au / NiO) in which gold was immobilized on nickel oxide was obtained by calcining at 300° C. in air for 4 hours. The average primary particle diameter of the obtain...
Embodiment 1-3
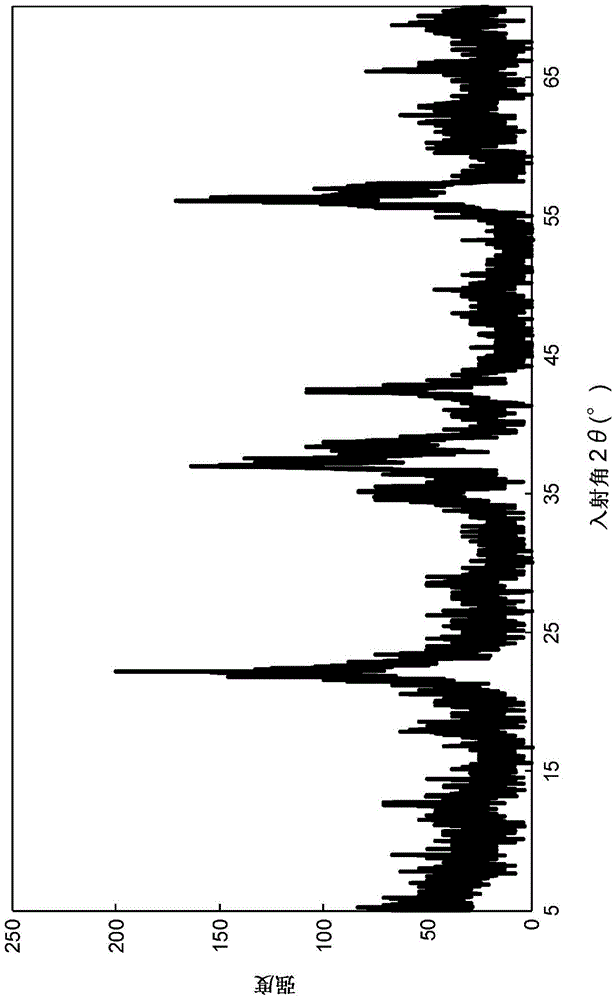
[0140] Example 1-3 (A solid catalyst with gold immobilized on iron oxide (hereinafter also referred to as "Au / Fe 2 o 3 ")Synthesis)
[0141] 7.68 g of iron (II) nitrate nonahydrate and 0.41 g of tetrachloroauric acid tetrahydrate were dissolved in 200 mL of distilled water at room temperature (aqueous solution 5). On the other hand, 3.88 g of sodium carbonate was separately dissolved in 370 mL of distilled water, and heated to 70° C. (aqueous solution 6). Next, the above-mentioned aqueous solution 5 was added to the above-mentioned aqueous solution 6 at one time, and the mixed solution was stirred at 70° C. for 1 hour. The resulting precipitate was washed with distilled water until the pH became constant, and filtered. The filtrate was dried overnight at 80°C. After drying, it was calcined at 300° C. in air for 4 hours to obtain a solid catalyst (Au / Fe 2 o 3 ). The average primary particle diameter of the obtained solid catalyst was about 20 nm, the immobilized amount...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com