Halometasone cream and preparation method thereof
A halometasone cream and a halometasone technology are applied in the field of medicine and can solve the problems of easy generation of impurities, easy deterioration, poor stability of the cream and difficult storage, and the like, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
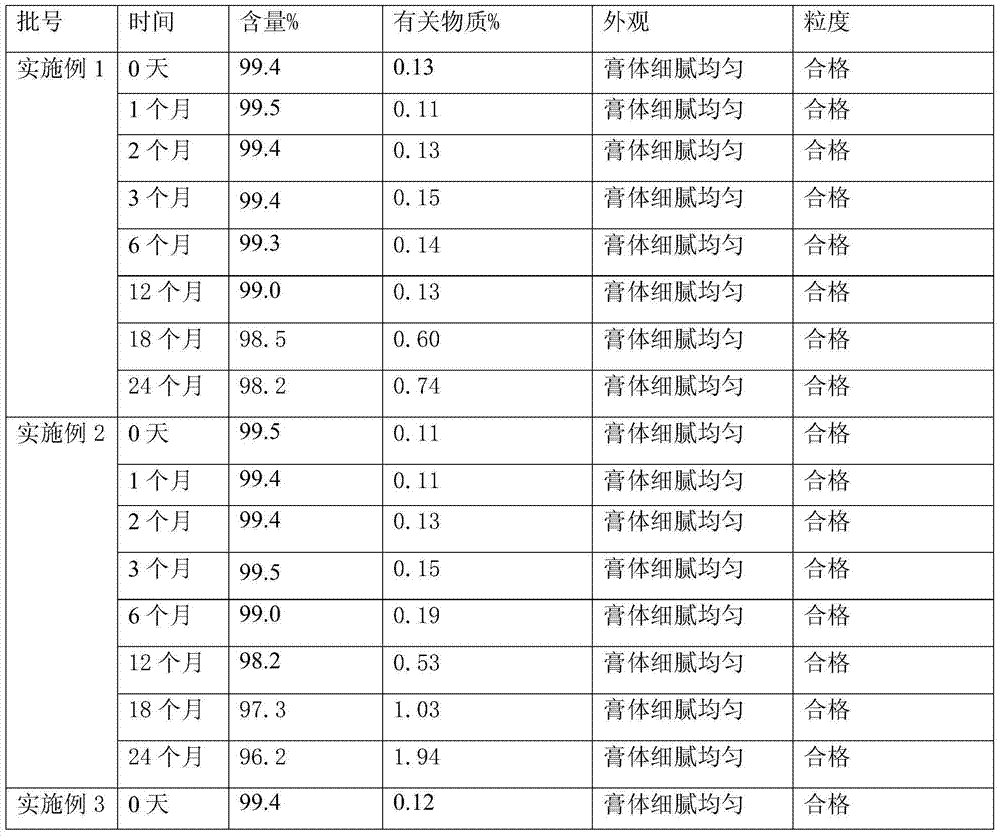
Embodiment 1-1
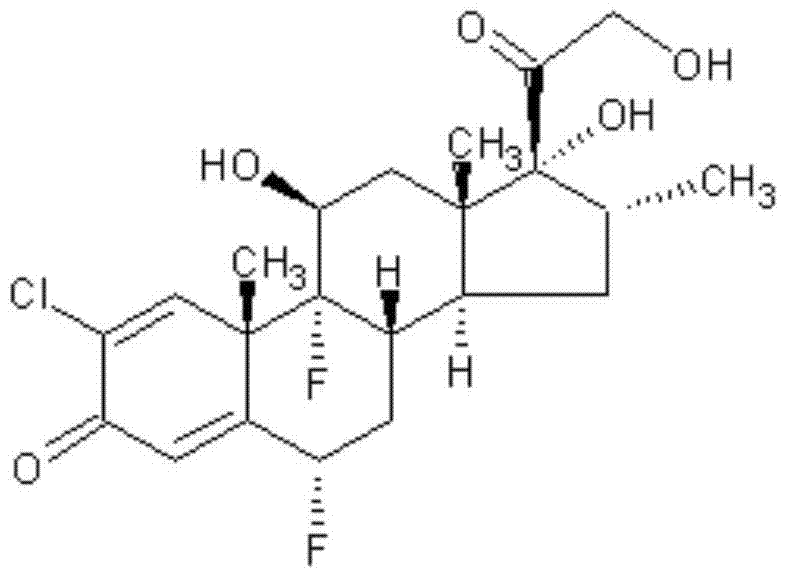
[0028] Prescription: halometasone monohydrate 0.5g, ascorbyl palmitate 0.5g, cetyl alcohol 45g, cetyl palmitate 40g, sodium lauryl sulfate 10g, disodium EDTA 1g, glycerin 60g gram, 10 grams of 2-phenoxyethanol, 55 grams of propylene glycol, 40 grams of stearylic acid, 45 grams of stearyl alcohol, 50 grams of white petrolatum, appropriate amount of water for injection, the total amount is up to 1000 grams.
[0029] preparation:
[0030] 1. Heat the propylene glycol to 45°C and keep it warm for later use;
[0031] 2. Weigh the halometasone hydrate and 2-phenoxyethanol of the prescribed amount, dissolve them in propylene glycol and keep warm for later use;
[0032] 3. Weigh cetyl alcohol, cetyl palmitate, octadecanoic acid, stearyl alcohol, white petrolatum, and ascorbyl palmitate in the prescribed amount, mix them, and heat to 98°C while stirring to melt. Get the oil phase and keep it warm for later use;
[0033] 4. Weigh sodium lauryl sulfate, disodium EDTA, and glycerin and...
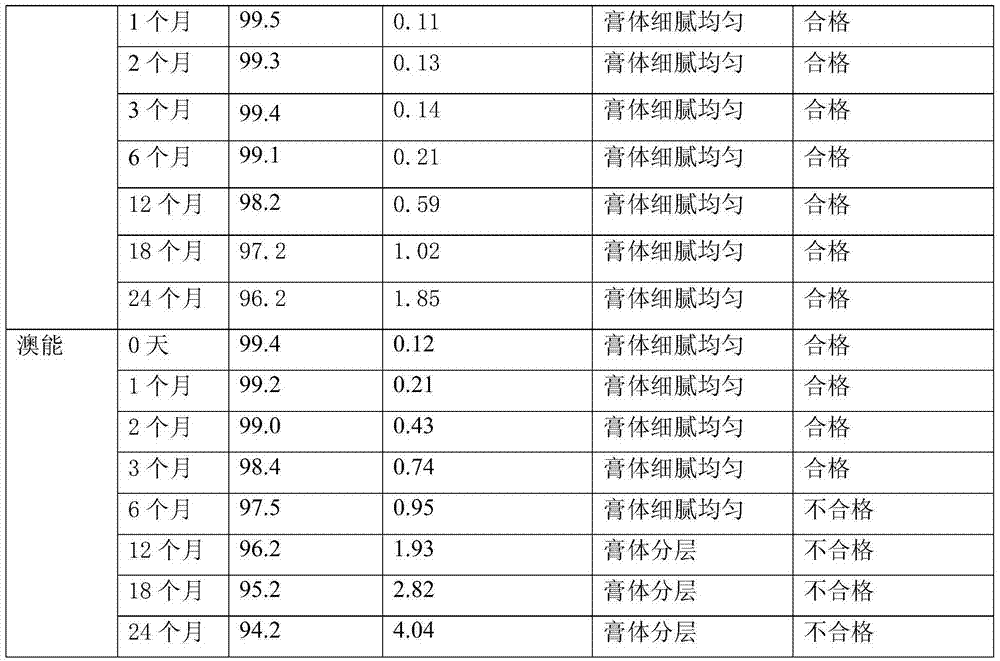
Embodiment 1-2
[0037] prescription:
[0038] Halometasone monohydrate 0.5g, ascorbyl palmitate 0.45g, cetyl alcohol 40.5g, cetyl palmitate 36g, sodium lauryl sulfate 9g, disodium EDTA 0.9g, glycerin 54g, 9 grams of 2-phenoxyethanol, 49.5 grams of propylene glycol, 36 grams of octadecanoic acid, 40.5 grams of stearyl alcohol, 45 grams of white petrolatum, appropriate amount of water for injection, the total amount is up to 1000 grams.
[0039] Preparation: The preparation method is the same as in Example 1-1.
Embodiment 1-3
[0041] Prescription: halometasone monohydrate 0.5g, ascorbyl palmitate 0.55g, cetyl alcohol 49.5g, cetyl palmitate 44g, sodium lauryl sulfate 11g, disodium EDTA 1.1g, glycerin 66 gram, 11 grams of 2-phenoxyethanol, 60.5 grams of propylene glycol, 44 grams of octadecanoic acid, 49.5 grams of stearyl alcohol, 55 grams of white petrolatum, appropriate amount of water for injection, the total amount is up to 1000 grams.
[0042] Preparation: The preparation method is the same as in Example 1-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com