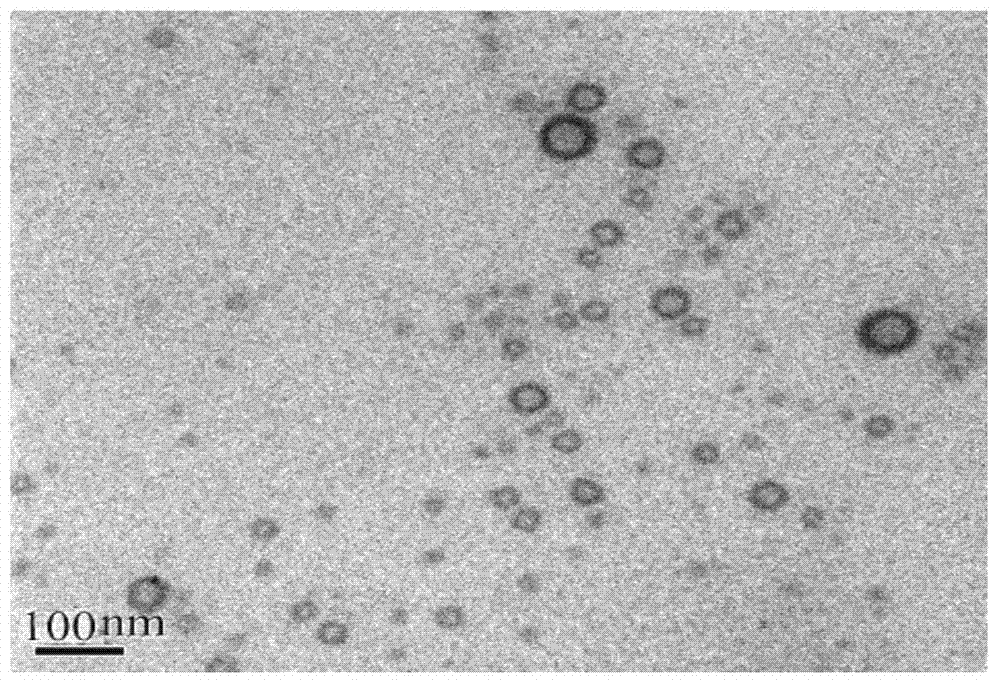
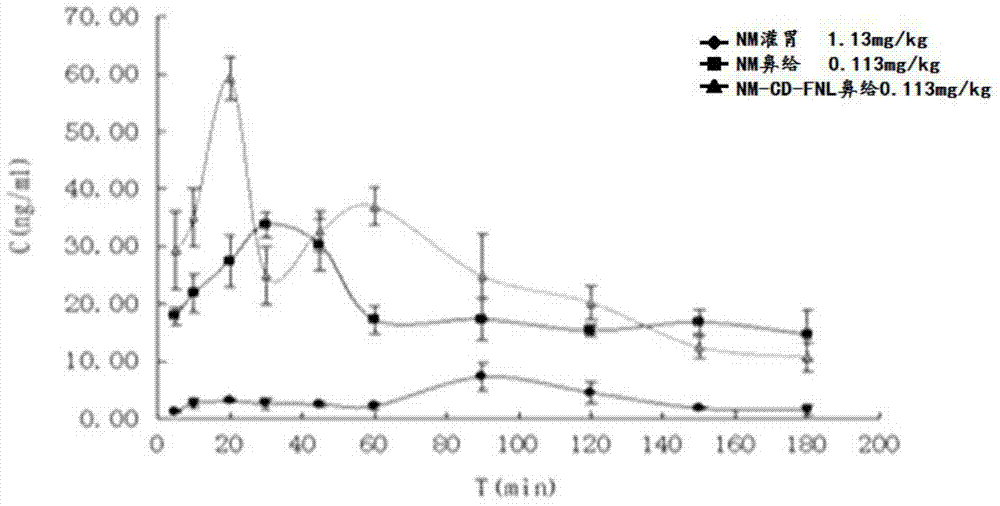
Nimodipine liposome for nasal delivery and preparation method thereof
A technology for nimodipine lipid and intranasal administration, applied in the field of pharmaceutical preparations, can solve problems such as limited improvement of drug absorption, and achieve the effects of avoiding or reducing irritant toxicity, low irritation, and improving therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
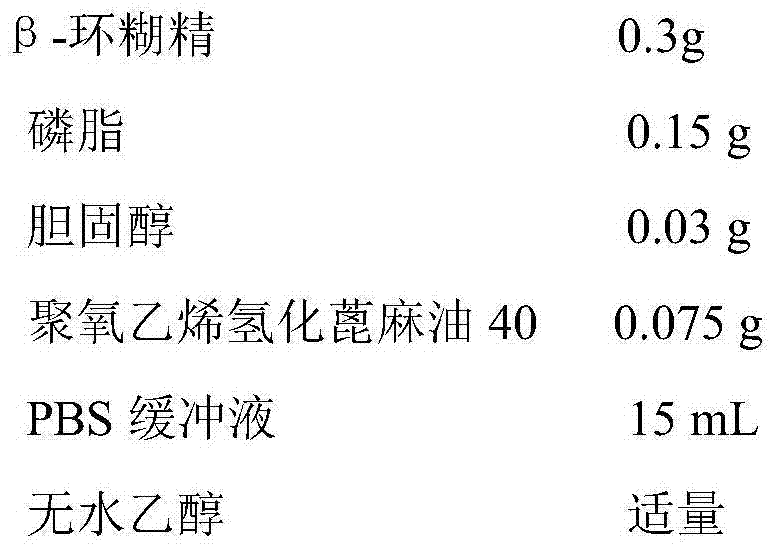
[0033] 1. Prescription:
[0034] Nimodipine-β-cyclodextrin inclusion compound 0.025g (the mass ratio of nimodipine to β-cyclodextrin is 1:3)
[0035]
[0036]
[0037] Second, the preparation method:
[0038] 1. Preparation of nimodipine-β-cyclodextrin inclusion compound by saturated aqueous solution method
[0039] Weigh 0.1g of nimodipine and 0.3g of β-cyclodextrin respectively, dissolve nimodipine in an appropriate amount of absolute ethanol (1ml) at 50°C, add β-cyclodextrin into distilled water to make a saturated aqueous solution; The solution was slowly added dropwise to a saturated aqueous solution of β-cyclodextrin, stirred in a water bath at 50°C for 3 hours; stored at 4°C overnight, filtered with suction, washed with water, washed with an appropriate amount of absolute ethanol to remove residual nimodipine, and dried at 40°C for 1 hour , that is, nimodipine-β-cyclodextrin inclusion compound (NM-β-CD).
[0040] 2. Preparation of nimodipine inclusion complex f...
Embodiment 2
[0043] 1. Prescription
[0044] Nimodipine-β-cyclodextrin inclusion compound 0.025g (the mass ratio of nimodipine to β-cyclodextrin is 1:10)
[0045]
[0046] Second, the preparation method:
[0047] 1. Preparation of nimodipine-β-cyclodextrin inclusion compound by saturated aqueous solution method
[0048] Weigh 0.1g of nimodipine and 1.0g of β-cyclodextrin respectively, dissolve nimodipine in an appropriate amount of absolute ethanol (1ml) at 40°C, add β-cyclodextrin into distilled water to make a saturated aqueous solution; The solution was slowly added dropwise to the saturated aqueous solution of β-cyclodextrin, stirred in a water bath at 40°C for 1 hour; stored at 4°C overnight, filtered with suction, washed with water, washed with an appropriate amount of absolute ethanol to remove residual nimodipine, and dried at 40°C for 1 hour , that is, nimodipine-β-cyclodextrin inclusion compound (NM-β-CD).
[0049] 2. Preparation of nimodipine inclusion complex flexible nan...
Embodiment 3
[0052] 1. Prescription:
[0053] Nimodipine-β-cyclodextrin inclusion compound 0.025g (the mass ratio of nimodipine to β-cyclodextrin is 1:1)
[0054]
[0055] Second, the preparation method:
[0056] 1. Preparation of nimodipine-β-cyclodextrin inclusion compound by saturated aqueous solution method
[0057] Weigh 0.1g of nimodipine and 0.1g of β-cyclodextrin respectively, dissolve nimodipine in an appropriate amount of absolute ethanol (1ml) at 65°C, add β-cyclodextrin into distilled water to make a saturated aqueous solution; The solution was slowly added dropwise to a saturated aqueous solution of β-cyclodextrin, stirred in a water bath at 65°C for 5 hours; stored at 4°C overnight, filtered with suction, washed with water, washed with an appropriate amount of absolute ethanol to remove residual nimodipine, and dried at 40°C for 1 hour , that is, nimodipine-β-cyclodextrin inclusion compound (NM-β-CD).
[0058] 2. Preparation of nimodipine inclusion complex flexible nano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com