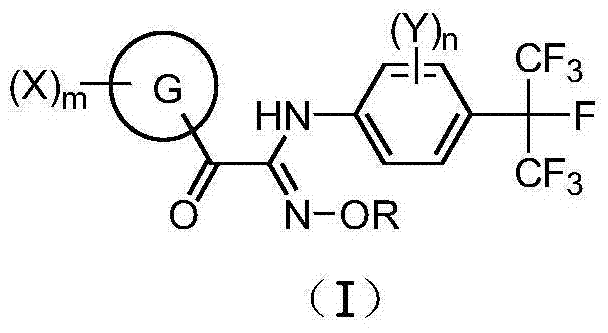
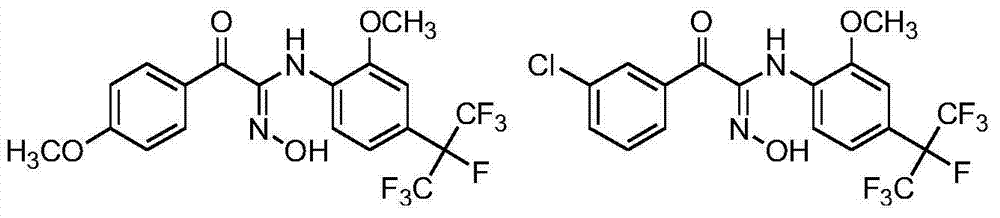
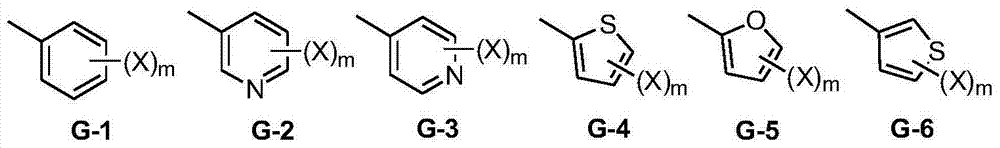Heptafluoroisopropyl-containing carbonyl oxime ether compound, preparation method and applications thereof
A technology of heptafluoroisopropyl and carbonyl oxime ether, which is applied in the direction of botany equipment and methods, applications, chemicals for biological control, etc., and can solve the problems that do not describe the use of insect pests and diseases, insecticidal and bactericidal activities No public issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Preparation of N-hydroxy-2-(3-chlorophenyl)-N’-[2-methoxy-4-heptafluoroisopropyl-phenyl]-2-oxo-acetamide (compound 60)
[0099] Step 1: Synthesis of m-chlorobenzene-α-chloro-α-carbonyl oxime
[0100]
[0101] Weigh 0.1mol of m-chloroacetophenone and 150.0g of dry dioxane in a 250mL dry three-neck flask for ice bathing. Add a drying tube above the reaction bottle. When the temperature of the reaction system is around 0°C, feed hydrogen chloride gas and adjust the gas flow rate to stabilize at 2 to 3 bubbles per second. After the gas flow is stable, slowly drop in 0.3 mol of isoamyl nitrite to ensure that the temperature of the reaction system is below 5°C. After dropping, continue to ventilate the gas, and you will find that the color of the reaction solution changes from colorless to deep red, indicating that saturated hydrogen chloride gas is dissolved in the reaction solution. Stop the gas flow, let the reaction solution continue to react at 0-5°C for about 1 ho...
Embodiment 2
[0106] Preparation of N-hydroxyl-2-(4-methoxy-phenyl)-N'-[2-methoxy-4-heptafluoroisopropyl-phenyl]-2-oxo-acetamide (compound 26)
[0107] Step 1: Synthesis of p-methoxybenzene-α-chloro-α-carbonyl oxime
[0108]
[0109] Using p-methoxyacetophenone as the raw material, the experimental procedure is the same as that in Example 1 for the synthesis of intermediate chlorobenzene-α-chloro-α-carbonyl oxime to obtain a white solid with a yield of 84.8%, m.p.116-118°C; 1 H NMR (400MHz, DMSO-d 6 ), δ: 3.85(s,3H,OCH 3 ), 6.99 (d, 2H, J=8.80Hz, Ar-H), 7.90 (d, 2H, J=8.80Hz, Ar-H), 13.64 (s, 1H, OH).
[0110] Step 2: Synthesis of target compound
[0111]
[0112] Taking p-methoxybenzene-α-chloro-α-carbonyl oxime and 2-methoxy-4-heptafluoroisopropylaniline as raw materials, the experimental procedure is the same as the synthesis of compound 60 in step 2 of Example 1 to obtain light Yellow solid, yield 84.3%.
Embodiment 3
[0114] N-(4-chlorobenzyloxy)-N'-(2,6-dimethyl-4-heptafluoroisopropyl-phenyl)-2-furan-2-yl-2-oxo-acetamide Preparation of (Compound 103)
[0115] Step 1: Synthesis of 2-furyl-α-chloro-α-carbonyl oxime
[0116]
[0117] Using 2-furanoacetophenone as the raw material, the experimental procedure was the same as the synthesis of intermediate chlorobenzene-α-chloro-α-carbonyl oxime in Example 1 to obtain a black solid with a yield of 81.8%, m.p.124-126°C; 1 H NMR (400MHz, DMSO-d 6 ), δ: 6.78 (m, 1H, Ar-H), 7.57 (d, 1H, Ar-H), 8.13 (d, 1H, Ar-H), 13.62 (s, 1H, OH).
[0118] Step 2: Synthesis of N-(2,6-dimethyl-4-heptafluoroisopropyl-phenyl)-2-furan-2-yl-N’-hydroxy-2-oxo-acetamide
[0119]
[0120] Using 2-furyl-α-chloro-α-carbonyl oxime and 2,6-dimethyl-4-heptafluoroisopropylaniline as raw materials, the experimental procedure is the same as the synthesis of compound 60 in step 2 of Example 1 to obtain Pale yellow solid, yield 82.1%.
[0121] Step 3: Synthesis of target co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com