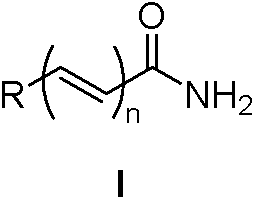
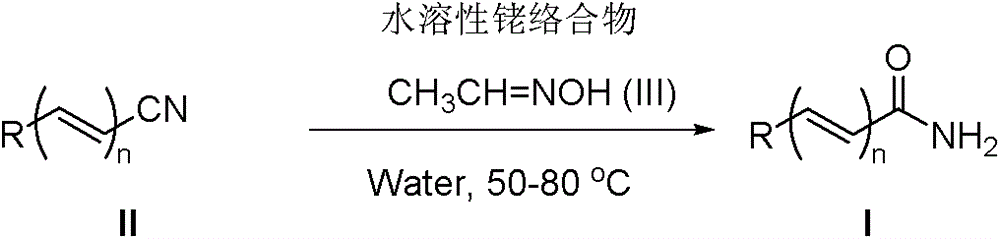
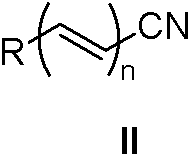A kind of method of nitrile hydrolysis synthetic amide
A technology of nitrile hydrolysis and amide, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, etc., to achieve the effects of low catalyst load, good energy saving effect, and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Benzamide
[0024]
[0025] Benzonitrile (103mg, 1mmol), [Cp*Rh(H 2 O) 3 ][OTf] 2 (3.0 mg, 0.005 mmol, 0.5 mol%), acetaldehyde oxime (65 mg, 1.1 mmol) and water (1 ml) were sequentially added to a 25 ml Schlenk reaction flask. After the reaction mixture was reacted at 50°C for 6 hours, it was cooled to room temperature, water was removed by rotary evaporation, and the target product was obtained by column separation, yield: 87%
[0026] 1 H NMR (500MHz, CDCl 3 )δ7.82 (d, J=7.3Hz, 2H, ArH), 7.54-7.44 (m, 3H, ArH), 6.09 (br s, 2H, NH 2 ); 13 C NMR (125MHz, CDCl 3 )δ169.7, 133.4, 131.9, 128.5, 127.3.
Embodiment 2
[0027] Example 2: 4-Methylbenzamide
[0028]
[0029]
[0030] 4-Methylbenzonitrile (117mg, 1mmol), [Cp*Rh(H 2 O) 3 ][OTf] 2 (3.0 mg, 0.005 mmol, 0.5 mol%), acetaldehyde oxime (65 mg, 1.1 mmol) and water (1 ml) were sequentially added to a 25 ml Schlenk reaction flask. After the reaction mixture was reacted at 50°C for 6 hours, it was cooled to room temperature, water was removed by rotary evaporation, and the target product was obtained by column separation, yield: 87%
[0031] 1 H NMR (500MHz, DMSO-d 6 )δ8.13 (br s, 1H, NH), 8.02-7.97 (m, 4H, ArH), 7.55 (br s, 1H, NH), 3.87 (s, 3H, CH 3 ); 13 C NMR (125MHz, DMSO-d 6 )δ168.0, 141.1, 131.5, 128.7, 127.5, 20.9.
Embodiment 3
[0032] Example 3: 3,5-Dimethylbenzamide
[0033]
[0034] 3,5-Dimethylbenzonitrile (131mg, 1mmol), [Cp*Rh(H 2 O) 3 ][OTf] 2 (3.0 mg, 0.005 mmol, 0.5 mol%), acetaldehyde oxime (65 mg, 1.1 mmol) and water (1 ml) were sequentially added to a 25 ml Schlenk reaction flask. After the reaction mixture was reacted at 50°C for 6 hours, it was cooled to room temperature. , rotary evaporation to remove water, column separation to obtain the target product, yield: 84%
[0035] 1 H NMR (500MHz, DMSO-d 6 )δ7.86(br s, 1H, NH), 7.46(s, 2H, ArH), 7.22(br s, 1H, NH), 7.14(s, 1H, ArH), 2.29(s, 6H, 2xCH 3 ); 13 C NMR (125MHz, DMSO-d 6 )δ168.6, 137.5, 134.3, 132.7, 125.4, 21.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com