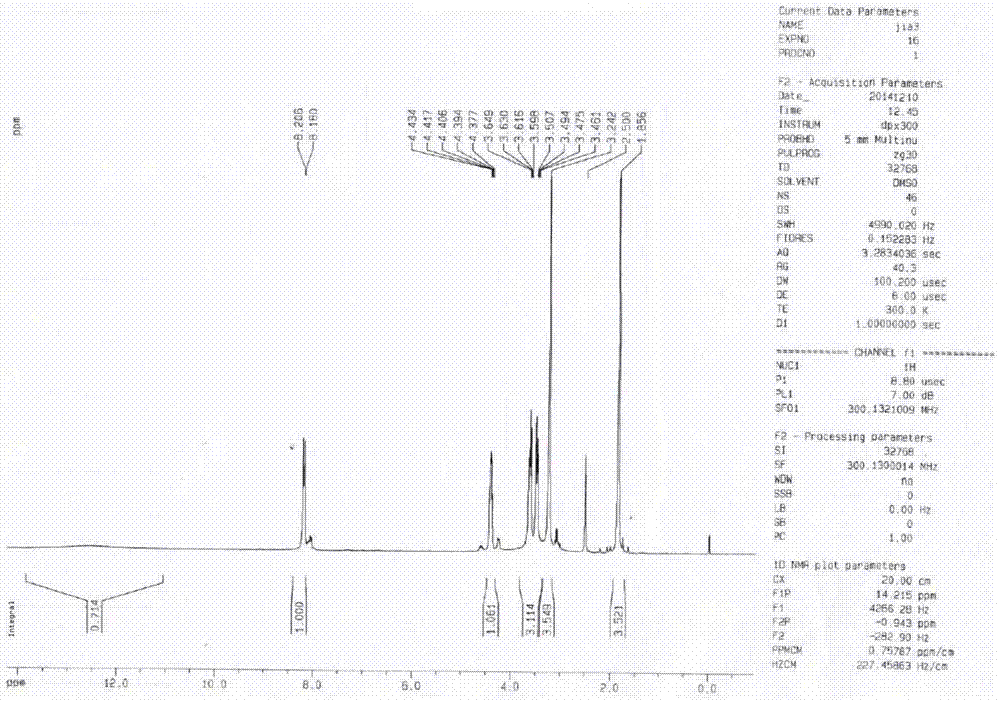
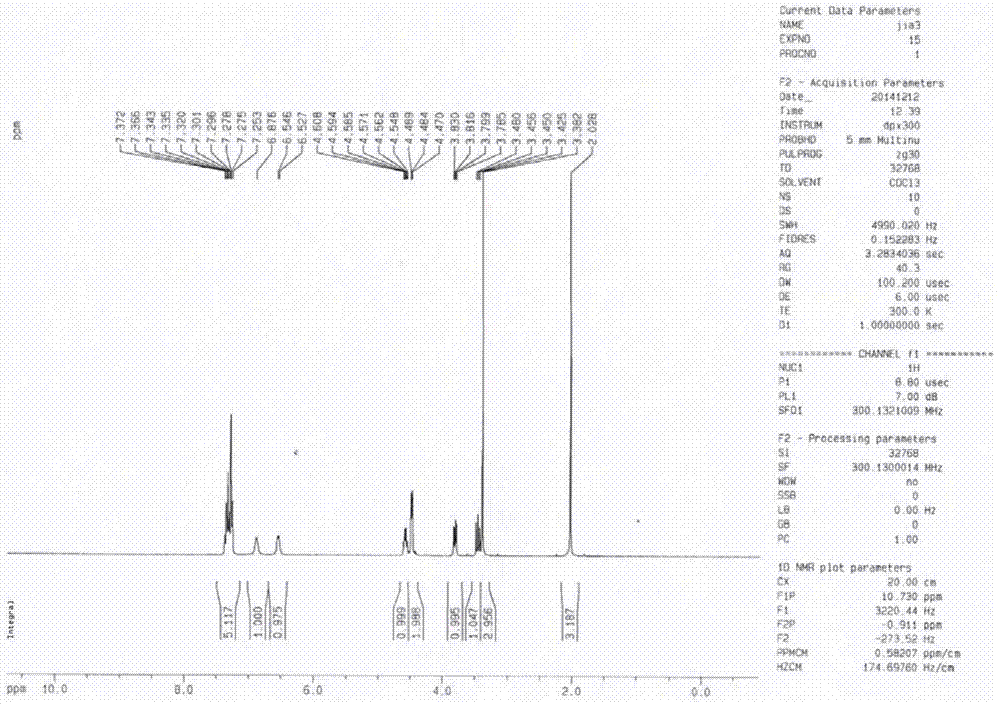
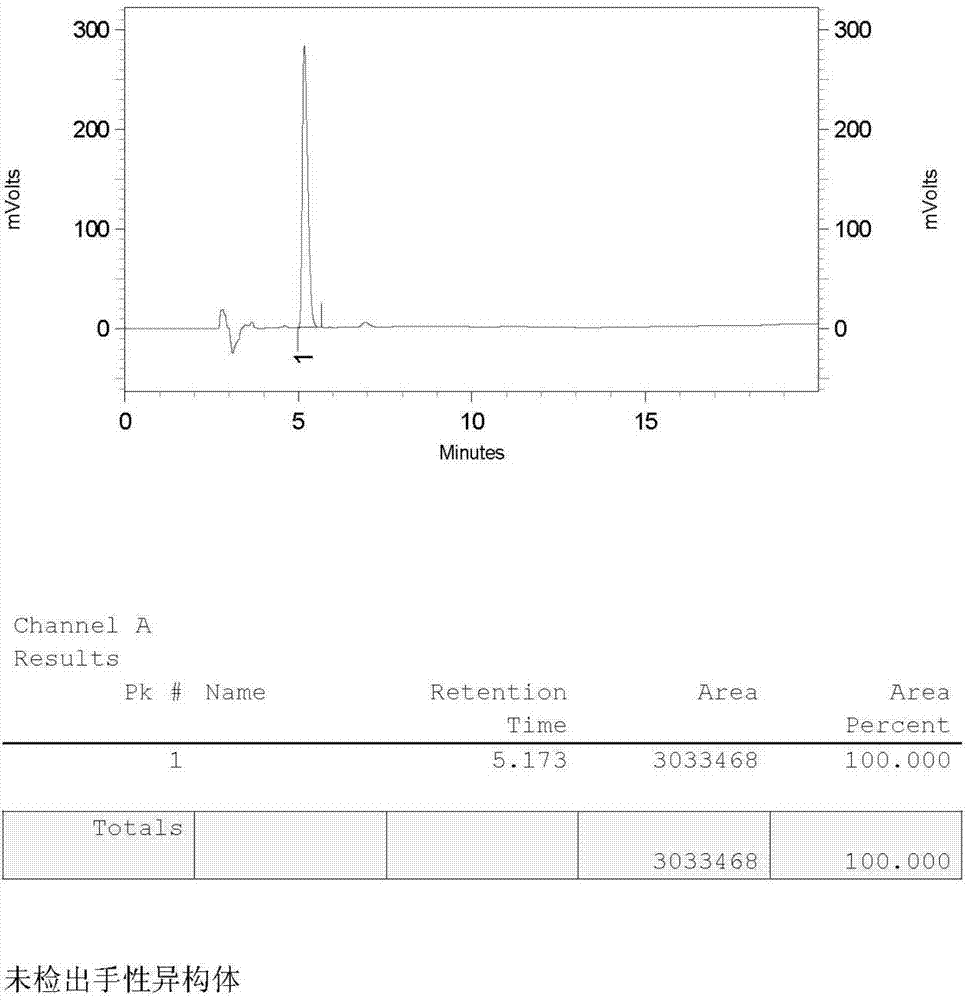
Preparation method of lacosamide
A technology of lacosamide and acetyl, which is applied in the field of preparation of lacosamide, can solve the problems of tediousness, long reaction route, and the total yield is only about 30-40%, and achieve simplified operation steps, stable product quality, and omission of The effect of the processing step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation method of lacosamide is as follows:
[0046] (1) Preparation of N-acetyl-D-serine methyl ether with D-serine
[0047] Add 100g of D-serine, 300mL of water to a 500mL three-neck flask, dropwise add 243.07g of acetic anhydride, react at 25-35°C for 2-3h, TLC analysis shows that the residual D-serine is less than 3%; add dropwise 10mol / L of sodium hydroxide Solution 300mL, 25-35 ℃ heat preservation reaction 3-4h, TLC analysis N, O-diacetyl-D-serine is less than 2%, lower the temperature to 10-15 ℃, drop 180.2g dimethyl sulfate, react 3-5h, The reaction was complete; acidified with hydrochloric acid to PH 2-3, extracted three times with dichloromethane, washed the dichloromethane layer with water, dried over anhydrous magnesium sulfate for 3 hours, and concentrated to dryness under reduced pressure to obtain a light yellow oil, which was kept in a freezer for 24 hours. Obtained 133.5g of white solid, yield 86.97%, HPLC: 97.3%, the compound confirmation spec...
Embodiment 2
[0051] The preparation method of lacosamide is as follows:
[0052] (1) Preparation of N-acetyl-D-serine methyl ether with D-serine
[0053] Add 100g D-serine, 300mL water to a 500mL three-necked flask, drop 200g acetic anhydride, react at 25-35°C for 2-3h, TLC analysis shows that the residual D-serine is less than 3%; add 10mol / L sodium hydroxide solution dropwise 300mL, keep warm at 25-35°C for 3-4h, TLC analysis N, O-diacetyl-D-serine is less than 2%, cool down to 10-15°C, add 170g trimethyl sulfate dropwise, react for 3-5h, the reaction is complete ; Acidify with hydrochloric acid to PH 2-3, extract three times with chloroform, wash the chloroform layer with water, dry over anhydrous magnesium sulfate for 3 hours, and concentrate to dryness under reduced pressure to obtain a light yellow oil, which is left in the freezer for 24 hours to obtain a white Solid 122.3g, yield 79.67%, HPLC: 96.0%;
[0054] (2) Preparation of Lacosamide
[0055] Add 800mL of chloroform to a 10...
Embodiment 3
[0057] The preparation method of lacosamide is as follows:
[0058] (1) Preparation of N-acetyl-D-serine methyl ether with D-serine
[0059] Add 100g D-serine, 300mL water, dropwise add 300g acetic anhydride to a 500mL three-neck flask, react at 25-35°C for 2-3h, TLC analysis shows that the residual D-serine is less than 3%; add dropwise 10mol / L potassium hydroxide solution 300mL, keep warm at 25-35°C for 3-4h, TLC analysis N, O-diacetyl-D-serine is less than 2%, cool down to 10-15°C, add 170g trimethyl sulfate dropwise, react for 3-5h, the reaction is complete Acidify with hydrochloric acid to PH 2-3, extract the mixture of chloroform and tetrahydrofuran three times, wash the layer of chloroform and tetrahydrofuran with water, dry over anhydrous magnesium sulfate for 3 hours, and concentrate to dryness under reduced pressure to obtain a light yellow oil, which is placed on After 24 hours in the freezer, 111.2 g of white solid was obtained, with a yield of 72.44%, HPLC: 94.8%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com