Lacosamidesolid preparation and preparation method thereof
A solid preparation, lacosamide technology, applied in the field of medicine, can solve the problems of low dissolution rate of preparations, many granulated fine powders, poor fluidity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 (1000 pieces amount)
[0024] Lacosamide 100g
[0025] Microcrystalline Cellulose PH102 24g
[0026] Croscarmellose Sodium 24g
[0027] Hypromellose 2.4g
[0028] Crospovidone CL 24g
[0029] Optimized Microcrystalline Cellulose 60g
[0030] Colloidal silicon dioxide 2.4g
[0031] Magnesium Stearate 2.4g
[0032] Gastric soluble film coating premix 7.2g
[0033] making process:
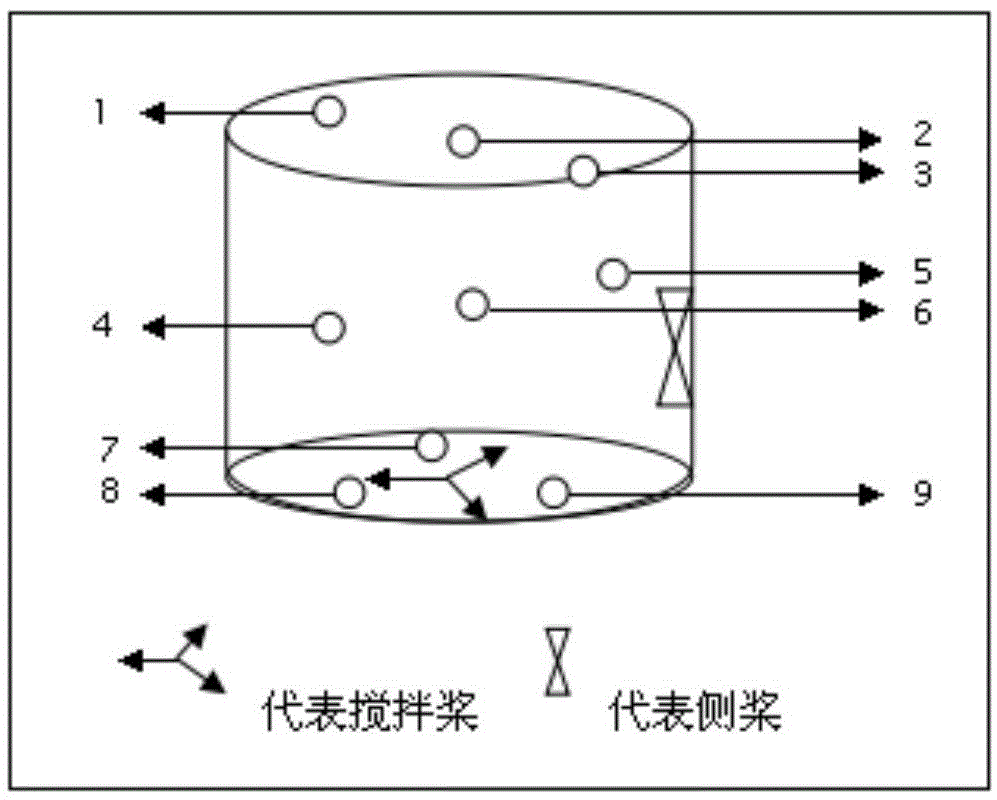
[0034] (1) Put the prescribed amount of lacosamide, microcrystalline cellulose PH102, and croscarmellose sodium in a wet mixer, and take three samples from the upper, middle, and lower parts of the mixer in 15 minutes, and measure the content , to calculate the content uniformity. Granulate and dry to obtain Mixture 1.
[0035] (2) Add optimized microcrystalline cellulose, crospovidone CL, colloidal silicon dioxide and magnesium stearate to mixture 1, and mix them together.
[0036] (3) Compression, coating and packaging.
Embodiment 2
[0037] Embodiment 2 (quantity of 1000 pieces)
[0038] Lacosamide 100g
[0039] Microcrystalline Cellulose PH102 36g
[0040] Croscarmellose Sodium 12g
[0041] Hypromellose 2.4g
[0042] Crospovidone CL 12g
[0043] Optimized Microcrystalline Cellulose 72g
[0044] Colloidal silicon dioxide 2.4g
[0045] Magnesium Stearate 2.4g
[0046] Gastric soluble film coating premix 7.2g
[0047] making process:
[0048] (1) Put the prescribed amount of lacosamide, microcrystalline cellulose PH102, and croscarmellose sodium in a wet mixer, and take three samples from the upper, middle, and lower parts of the mixer in 15 minutes, and measure the content , to calculate the content uniformity. Granulate and dry to obtain Mixture 1.
[0049] (2) Add optimized microcrystalline cellulose, crospovidone CL, colloidal silicon dioxide and magnesium stearate to mixture 1, and mix them together.
[0050] (3) Compression, coating and packaging.
Embodiment 3
[0051] Embodiment 3 (quantity of 1000 pieces)
[0052] Lacosamide 100g
[0053] Microcrystalline Cellulose PH102 30g
[0054] Croscarmellose Sodium 18g
[0055] Hypromellose 2.4g
[0056] Crospovidone CL 18g
[0057] Optimized Microcrystalline Cellulose 66g
[0058] Colloidal silicon dioxide 2.4g
[0059] Magnesium Stearate 2.4g
[0060] Gastric soluble film coating premix 7.2g
[0061] making process:
[0062] (1) Put the prescribed amount of lacosamide, microcrystalline cellulose PH102, and croscarmellose sodium in a wet mixer, and take three samples from the upper, middle, and lower parts of the mixer in 15 minutes, and measure the content , to calculate the content uniformity. Granulate and dry to obtain Mixture 1.
[0063] (2) Add optimized microcrystalline cellulose, crospovidone CL, colloidal silicon dioxide and magnesium stearate to mixture 1, and mix them together.
[0064] (3) Compression, coating and packaging.
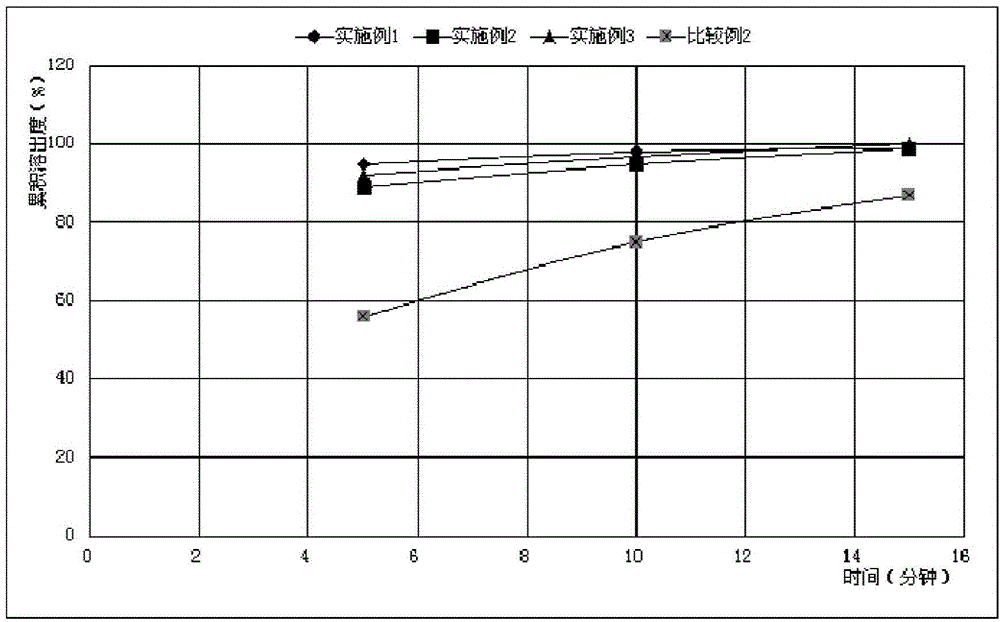
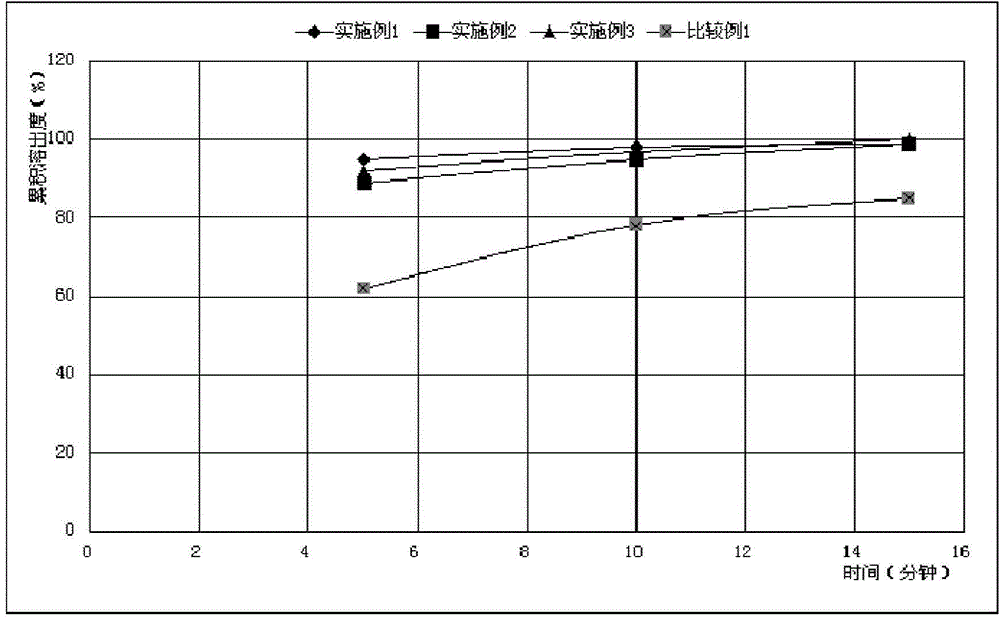
[0065] For the convenience of comparison ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com