Chiral (2,2-dimethyl-1,3-dioxolane-4-yl)hydroxy mesylates, and preparing method and applications thereof
A technology of hydroxymethanesulfonate and dioxolane, which is applied in the field of chiral hydroxymethanesulfonate and its preparation, can solve the problems of not being able to store for a long time, solve the difficulties of transportation and storage, and facilitate long-term storage and transshipment , the effect of structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
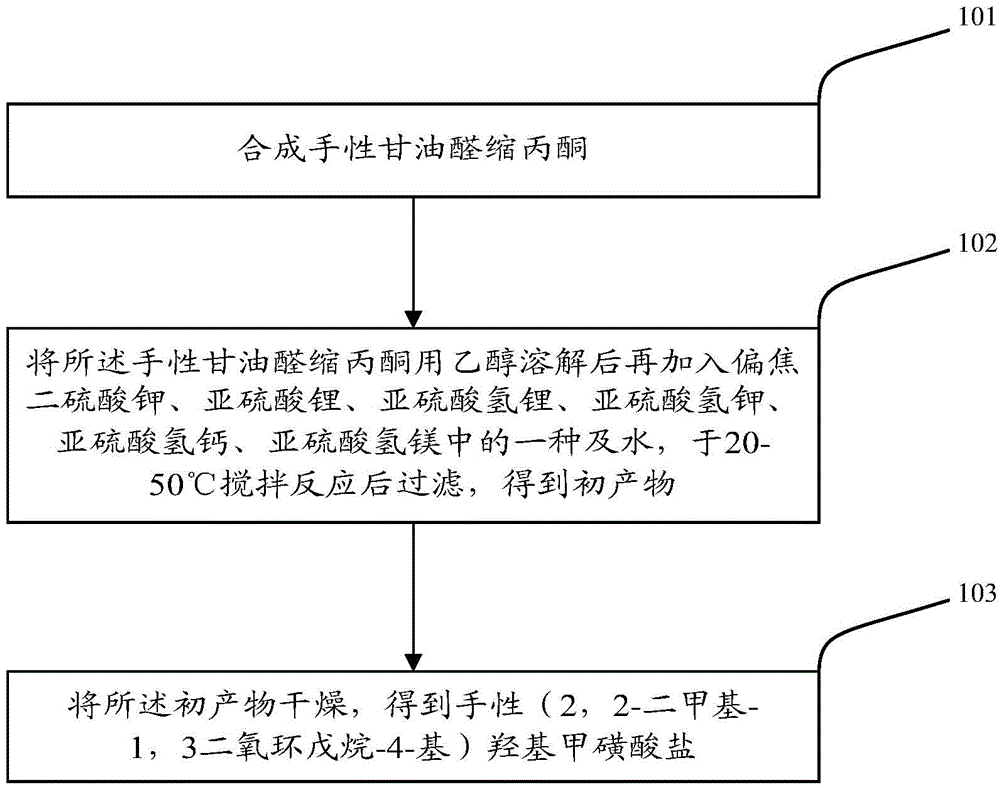
[0051] Please refer to figure 1 , the preparation method of the above-mentioned chiral (2,2-dimethyl-1,3-dioxolan-4-yl) hydroxymethanesulfonate that the present invention also provides comprises the following steps:
[0052] Step 101: synthesizing chiral glyceraldehyde acetonide;
[0053] Preferably, due to the unstable structure of chiral glyceraldehyde acetone, in order to improve its yield, preferably, this step can be specifically carried out according to the following operations:
[0054] S1: Mix 1,2,5,6-dipropylidene-D-mannitol and dichloromethane, stir and dissolve at 15-25°C, then add saturated bicarbonate and sodium periodate in sequence, and continue to stir for reaction 1 After -4 hours, add anhydrous sodium sulfate / anhydrous magnesium sulfate to dry to obtain a primary mixture;
[0055] In step S1, the amount of each reactant is preferably defined as follows: the molar ratio of 1,2,5,6-dipropylidene-D-mannitol to dichloromethane is 1:(35-60); The molar ratio of ...
Embodiment 1
[0073] The preparation method of the chiral (2,2-dimethyl-1,3-dioxolan-4-yl) hydroxymethanesulfonate provided in the examples of the present invention comprises:
[0074] S11: Oxidation reaction
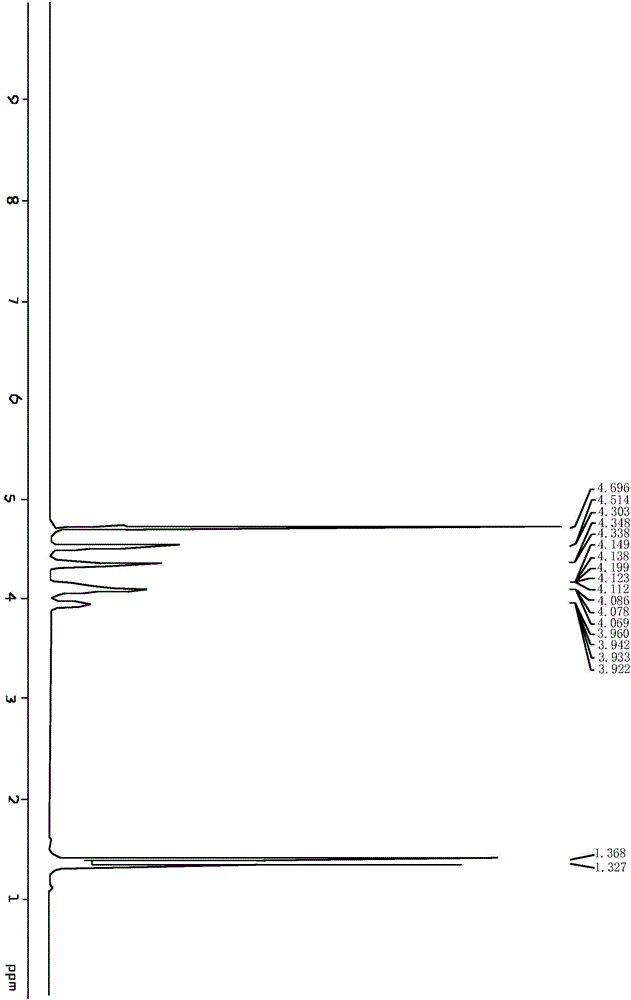
[0075] Add 1,2,5,6-dipropylidene-D-mannitol (1mol) and dichloromethane (40mol) into the reactor, stir and dissolve at 20°C, then add saturated sodium bicarbonate (0.1mol), Add sodium periodate (1.45mol) in batches under continuous stirring, and keep the reaction temperature below 35°C during the addition of sodium periodate. After adding sodium periodate, continue to stir and react at 25°C for 2h, then add anhydrous sulfuric acid Sodium (1mol) was dried for 60min., and anhydrous sodium sulfate was added, and the reaction temperature did not exceed 30°C. Filtrate to obtain (R) mannaldehyde acetonide dichloromethane solution, and recover the dichloromethane by rotary evaporation at 35-50° C. to obtain colorless and transparent viscous (R) glyceraldehyde acetone.
[0076] S12: Salt fo...
Embodiment 2
[0080] The preparation method of the chiral (2,2-dimethyl-1,3-dioxolan-4-yl) hydroxymethanesulfonate provided in the examples of the present invention comprises:
[0081] S21: Oxidation reaction
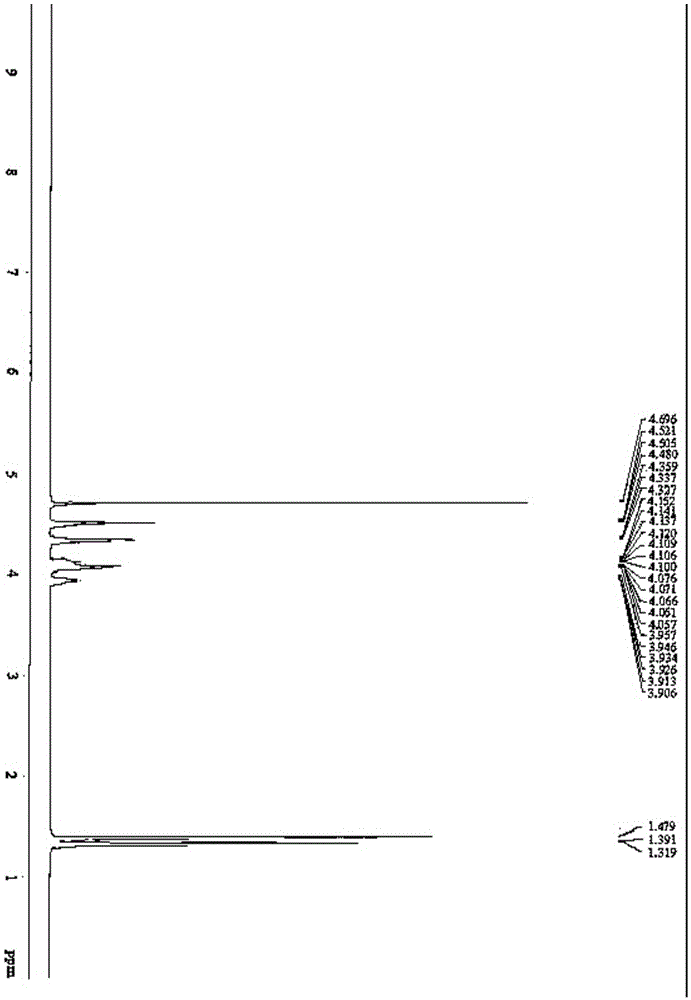
[0082] Add 1,2,5,6-dipropylidene-D-mannitol (1.5mol) and dichloromethane (65mol) into the reactor, stir and dissolve at 20°C, then add saturated potassium bicarbonate (0.16mol) , continue to stir and add sodium periodate (1.45mol) in batches, and keep the reaction temperature below 30°C during the addition of sodium periodate. After adding sodium periodate, continue stirring at 25°C for 1.5h, then add Magnesium sulfate water (1.2mol) was dried for 60 min., and anhydrous magnesium sulfate was added, and the reaction temperature did not exceed 30°C. Filtrate to obtain (R) mannaldehyde acetonide dichloromethane solution, and recover the dichloromethane by rotary evaporation at 40-50° C. to obtain light yellow transparent viscous (R) glyceraldehyde acetonide.
[0083] S22: Salt formati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com