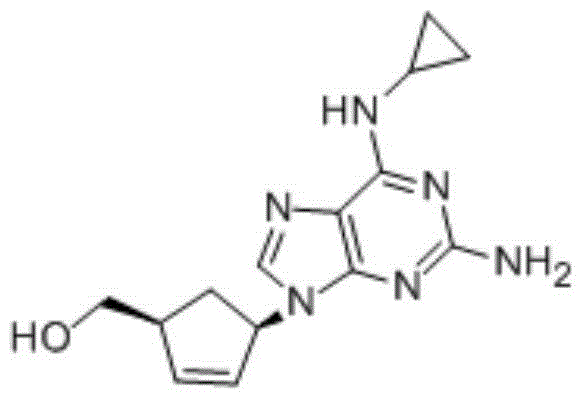
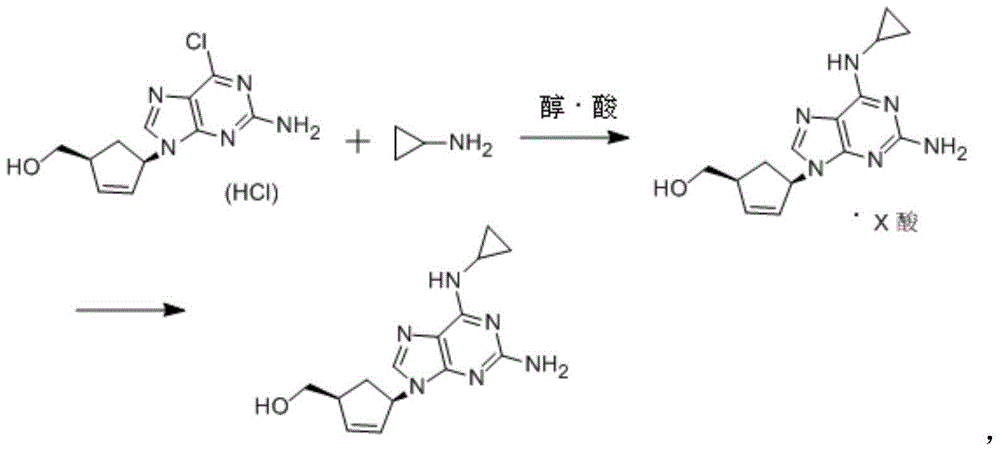
Preparation method of abacavir
A kind of amino, hydrochloride technology, applied in the field of medicine and chemical industry, can solve the problem of difficult to remove impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of abacavir dihydrochloride
[0039] (1S,4R)-4-(2-amino-6-chloro-9H-purin-9-yl)cyclopent-2-en-1-ylmethanol hydrochloride (30g, 0.1mol) and cyclopropylamine ( 22.6 g, 0.4 mol) into 180 g of ethanol, heated to reflux until the reaction was completed, then added sodium carbonate (11 g, 0.1 mol), evaporated part of the solvent, filtered while hot, concentrated the filtrate to dryness, added 200 g of fresh ethanol and heated to dissolve After clearing, cool down to 40-45°C, add 35.5% ethanol hydrochloride (20.5g, 0.2mol) dropwise, heat up to 50-55°C after dropping, keep warm for 0.5-1 hour, cool down to room temperature, and filter to obtain Abacavir dihydrochloride 32.6g, yield is 91.4%.
Embodiment 2
[0040] Embodiment 2: the preparation of abacavir dihydrobromide
[0041] (1S,4R)-4-(2-amino-6-chloro-9H-purin-9-yl)cyclopent-2-en-1-ylmethanol hydrochloride (30g, 0.1mol) and cyclopropylamine ( 22.6g, 0.4mol) into 180g of isopropanol, heated to reflux until the reaction was over, then put in sodium carbonate (11g, 0.1mol), evaporated part of the solvent, filtered while hot, concentrated the filtrate to dryness, and put in 200g of fresh isopropanol After the alcohol is heated until it dissolves, cool down to 40-45°C, add 25% isopropanol hydrobromide (64.8g, 0.2mol) dropwise, raise the temperature to 50-55°C after dropping, and keep warm for 0.5-1 hour , cooled to room temperature, and filtered to obtain 40.1 g of abacavir dihydrobromide, with a yield of 90.1%.
Embodiment 3
[0042] Embodiment 3: the preparation of abacavir dibenzoate
[0043] (1S,4R)-4-(2-amino-6-chloro-9H-purin-9-yl)cyclopent-2-en-1-ylmethanol hydrochloride (30g, 0.1mmol) and cyclopropylamine ( 22.6g, 0.4mol) into 180g of isopropanol, heated to reflux until the reaction is over, put in sodium carbonate (11g, 0.1mol), evaporate part of the solvent, filter while hot, concentrate the filtrate to dryness, put in 400g of fresh isopropanol After the alcohol is heated to dissolve, cool down to 40-45°C, add benzoic acid (24.4g, 0.2mol), raise the temperature to 50-55°C after dropping, keep warm for 0.5-1 hour, cool down to room temperature, filter to obtain Bacavir dibenzoate 48.76g, the yield is 92.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com