Duloxetine chiral intermediate mandelate preparation method
A technology of mandelic acid salt and mandelic acid, applied in directions such as carboxylate preparation, organic chemistry, etc., can solve the problems such as increased amount of resolving agent, lack of resolving agent recycling method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
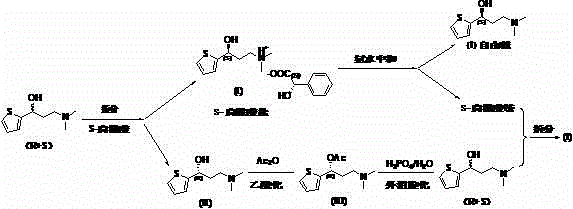
[0042] Embodiment 1: (R / S)-( + Preparation of )-N,N-dimethyl-3-(2-thienyl)-3-hydroxypropylamine
[0043]In a 500 mL four-necked flask, 25 g of 2-thiophene-2-dimethylaminomethyl ethyl ketone hydrochloride (0.114 mol), 150 mL of ethanol and 75 mL of water were added to dissolve them all. Stir at room temperature, slowly add 13.7g 30% NaOH solution, adjust the pH to 11-12, add 3.0 g (0.08mol) sodium borohydride in portions under temperature control below 10°C, and stir overnight at room temperature. Add 5mL of acetone, stir for 20min, distill off the ethanol under reduced pressure, a white solid precipitates out, add 150ml of tert-butyl methyl ether to the residue, stir, extract and separate layers, extract the water layer with 50ml of tert-butyl methyl ether, discard it, combine the organic layers, and brine It was directly used in Example 2 after washing.
[0044]
Embodiment 2
[0045] Embodiment 2: (R / S)-( + Resolution of )-N,N-Dimethyl-3-(2-thienyl)-3-hydroxypropylamine
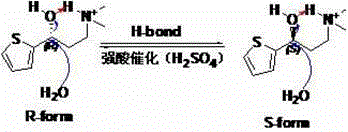
[0046] In the 100mL conical flask, add 10.4g (68.4mmol) (S)-mandelic acid, be dissolved in 40ml ethanol at 50 ℃, then the solution of mandelic acid is slowly added dropwise to the (R / S)-( + )-N,N-Dimethyl-3-(2-thienyl)-3-hydroxypropylamine tert-butyl methyl ether solution, stirred and gradually precipitated a white solid, then cooled to 0~5°C in an ice bath and stirred for 1h, filtered , The filter cake was recrystallized with absolute ethanol 75m. After drying, 15.7 g of (S)-(-)-N,N-dimethyl-3-(2-thienyl)-3-hydroxypropylamine S-mandelic acid salt (I) was obtained, with a yield of 40.9%.
[0047] The optical purity was determined by high performance liquid chromatography (HPLC, chiral column), S-configuration: 98.5% R configuration: 1.5%.
[0048] After the two crystallization mother liquors were concentrated, 15.9g of oily matter was obtained, 100ml of tert-butyl methyl ether a...
Embodiment 3
[0051] Example 3: Preparation of (S)-(-)-N,N-dimethyl-3-(2-thienyl)-3-hydroxypropylamine (neutralization experiment 1)
[0052] 15.7g (47.1mmole) (S)-(-)-N,N-dimethyl-3-(2-thienyl)-3-hydroxypropylamine S-mandelic acid obtained in Example 2 was added to a 250ml four-necked bottle Salt (I) and 90ml of tert-butyl methyl ether, add 6.9g of 23% ammonia water (90.7mmole) under stirring, stir and neutralize the layers, and collect the organic layer for temporary storage. The water layer was concentrated to obtain a viscous substance, which was added with 30ml of ethanol and concentrated again with water, and the residue was dissolved in 20ml of ethanol for use in Example 4 as a resolving agent. The organic layer was washed with 10 ml of brine and then concentrated under reduced pressure to obtain 7.9 g of the white product (S)-(-)-N,N-dimethyl-3-(2-thienyl)-3-hydroxypropylamine with a yield of 91.6%. Chiral analysis: S-configuration: 98.7% R configuration: 1.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com