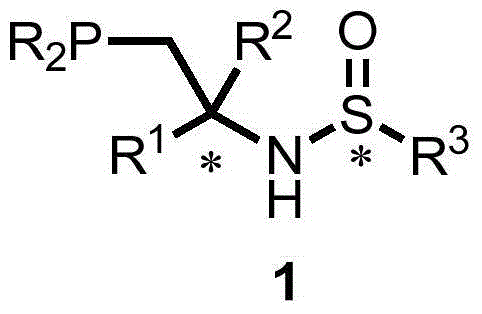
Chiral tertiary phosphine compound, full configuration, preparation method and application thereof
A compound, chiral phosphine technology, applied in chiral tertiary phosphine compound and its full configuration, its preparation and application fields, can solve the problems of expensive raw materials, low overall yield, long synthetic route, etc., and achieve easy transformation, raw material Inexpensive, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
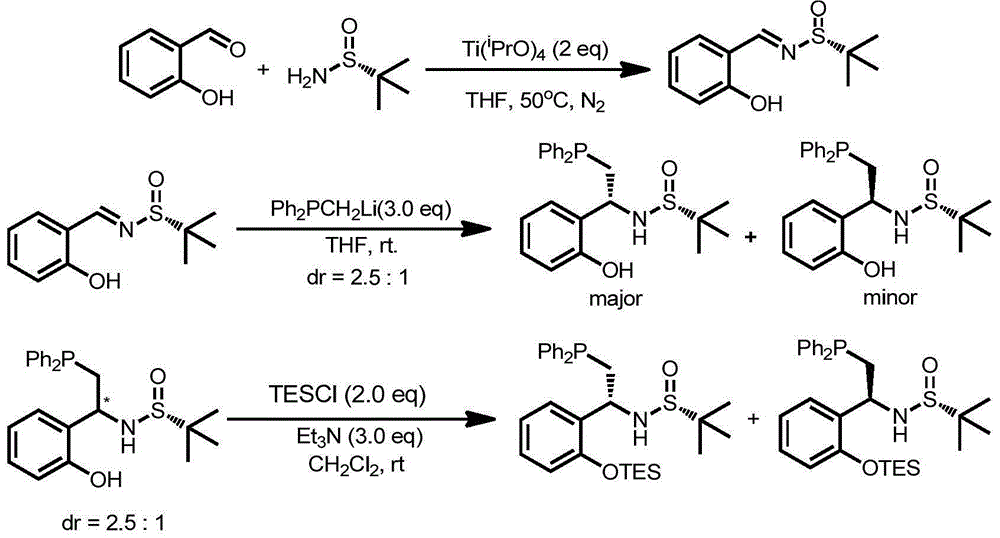
[0040] (S)-N-((R S )-2-(diphenylphosphino)-1-phenylethyl)-2-methylpropane-2-sulfinamide[1a(S,R S )]Synthesis
[0041]
[0042] Wherein, THF is tetrahydrofuran; N 2 is nitrogen; Ti( i PrO) 4 For tetraisopropyl titanate.
[0043] Step 1: In a 500mL three-necked flask, add benzaldehyde (50mmol) and (R)-(+)-tert-butylsulfinamide (50mmol), add 150mL tetrahydrofuran under nitrogen atmosphere, add tetratitanate Isopropyl ester (100mmol), stirred at 50°C for 10h, the yield was 85%.
[0044]
[0045] The second step: add the imine (1.04 g, 5 mmol) prepared in the first step into a 50 mL eggplant-shaped reaction flask, protect with nitrogen, and add 30 mL of tetrahydrofuran. Lithium diphenylmethylenephosphine (10 mmol) was slowly added at room temperature, stirred overnight, and the yield was 71%. Proton NMR (400MHz, CDCl 3 )δ7.45-7.41(m,2H),7.36-7.28(m,7H),7.27-7.25(m,6H),4.48-4.41(m,1H),3.63(d,J=4.4Hz,1H) ,2.89(dd,J=13.8,6.8Hz,1H),2.50(dd,J=13.8,8.0Hz,1H),1.19(s,9H).Car...
Embodiment 2
[0047] (S)-N-((R S )-2-(diphenylphosphino)-1-(naphthalen-2-yl)ethyl)-2-methylpropane-2-sulfinamide[1b(S,R S )]Synthesis.
[0048]
[0049] Refer to Example 1 for the specific operation, the raw material used is (R)-(+)-tert-butylsulfinamide, the nucleophile is lithium diphenylmethylenephosphine, and the total yield is 70%. Proton NMR (400MHz, CDCl 3 )δ7.79-7.75(m,3H),7.70(s,1H),7.46-7.39(m,5H),7.36-7.27(m,5H),7.21-7.18(m,3H),4.66-4.59( m,1H),3.71(d,J=4.3Hz,1H),2.98(dd,J=13.8,6.8Hz,1H),2.62(dd,J=13.8,8.0Hz,1H),1.20(s,9H ). Carbon NMR (100MHz, CDCl 3 )δ139.25(d,J C,P =4.4Hz), 138.02(d, J C,P =12.7Hz), 137.52(d, J C,P =12.9Hz), 133.13(d, J C,P =5.8Hz), 132.83(d, J C,P =3.1Hz), 132.64(d, J C,P =3.5Hz), 128.81, 128.62, 128.55, 128.32 (d, J C,P =6.7Hz), 128.02, 126.40(d, J C,P =1.1Hz), 126.11(d, J C,P =9.9Hz), 124.77, 57.73(d, J C,P =19.6Hz), 56.08, 37.16(d, J C,P =14.4Hz), 22.56; Phosphine NMR (162MHz, CDCl 3 )δ=-23.88ppm. High-resolution mass spectrometry theore...
Embodiment 3
[0051] (S)-N-((R S )-1-(diphenylphosphino)-3-methylbutan-2-yl)-2-methylpropane-2-sulfinamide[1c(S,R S )]Synthesis
[0052]
[0053] The specific operation refers to Example 1, the raw material used is (R)-(+)-tert-butylsulfinamide, the nucleophile is lithium diphenylmethylenephosphine, and the total yield is 78%. Proton NMR (400MHz, C 6 D. 6 )δ7.47-7.43(m,4H),7.16-7.03(m,6H),3.34-3.24(m,2H),2.25-2.16(m,3H),1.09(s,9H),0.87(d, J=6.8Hz, 3H), 0.72(d, J=6.9Hz, 3H). Carbon NMR (100MHz, C 6 D. 6 )δ139.79(d,J C,P =13.9Hz), 139.10(d, J C,P =15.3Hz), 133.62(d, J C,P =19.8Hz), 132.66(d, J C,P =18.3Hz), 129.08, 128.81(d, J C,P =6.9Hz), 128.74(d, J C,P =6.2Hz), 128.55, 60.41, 56.02, 33.44 (d, J C,P =6.9Hz), 32.24(d, J C,P =13.85Hz), 22.69, 18.27, 17.32; Phosphine NMR (162MHz, C 6 D. 6 )δ=-9.05ppm. High-resolution simple theoretical data C 21 h 31 NOPS:m / z(%):376.1858(M+H + ), experimental data: 376.1859.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com