Intein-modified proteases, their production and industrial applications
A protease and intein technology, which is applied in the field of protease and intein-modified protease, can solve the problems such as difficult production of enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0109] The following items include specific embodiments. However, it is not intended to limit or exclude the embodiments described elsewhere herein or alternative embodiments.
[0110] 1. An intein-modified protease comprising a target protease and an intein fused to the target protease in a position such that the intein can control the activity of the target protease , wherein the intein is capable of effecting intein-modified protease splicing.
[0111] 2. The intein-modified protease of embodiment 1, wherein the fusion is at a position such that the intein is capable of substantially reducing or inhibiting the activity of the target protease.
[0112] 3. The intein-modified protease according to any one or more of embodiments 1-2, wherein the target protease is selected from the group consisting of: EC3.4.99 protease, EC3.4.21.62 protease, keratinase, Enzymes in the group consisting of serine proteases, alkaline proteases, metalloproteases, cysteine proteases, aspartic ...
Embodiment 1
[0170] Embodiment 1 Experiment overview
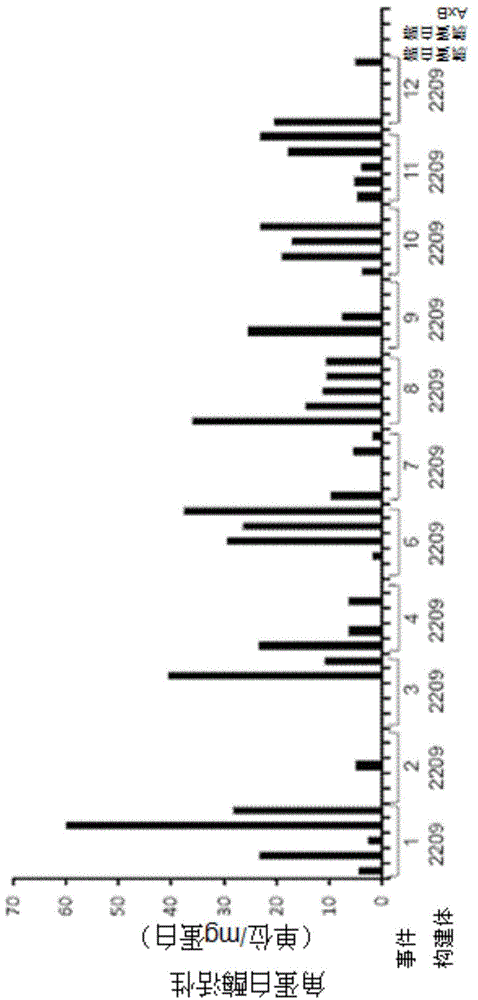
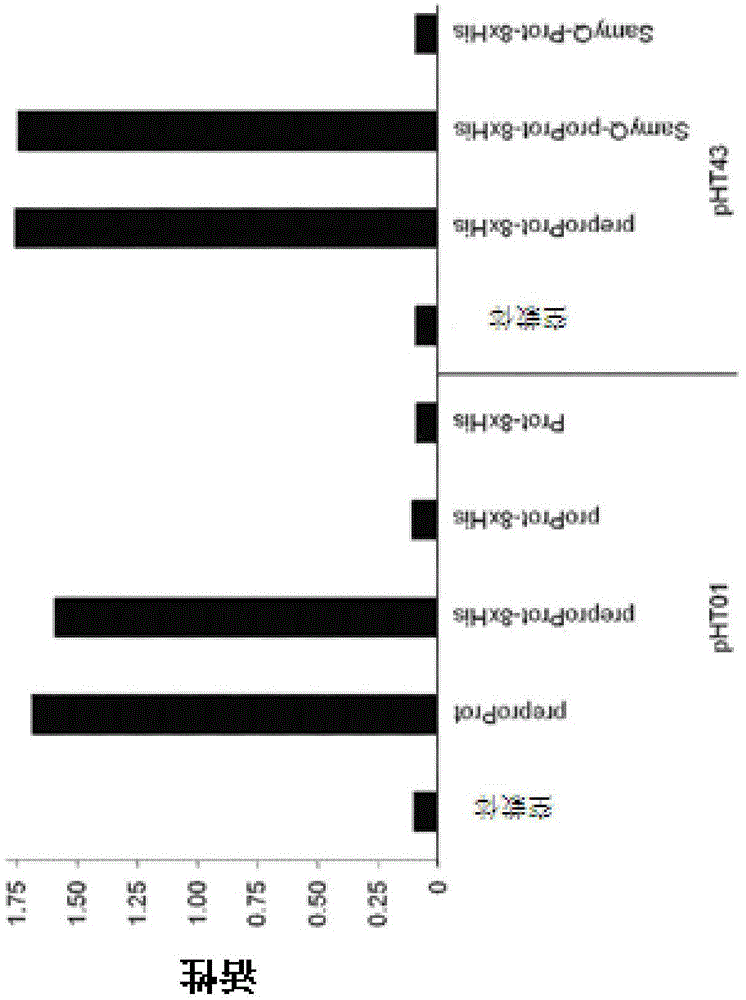
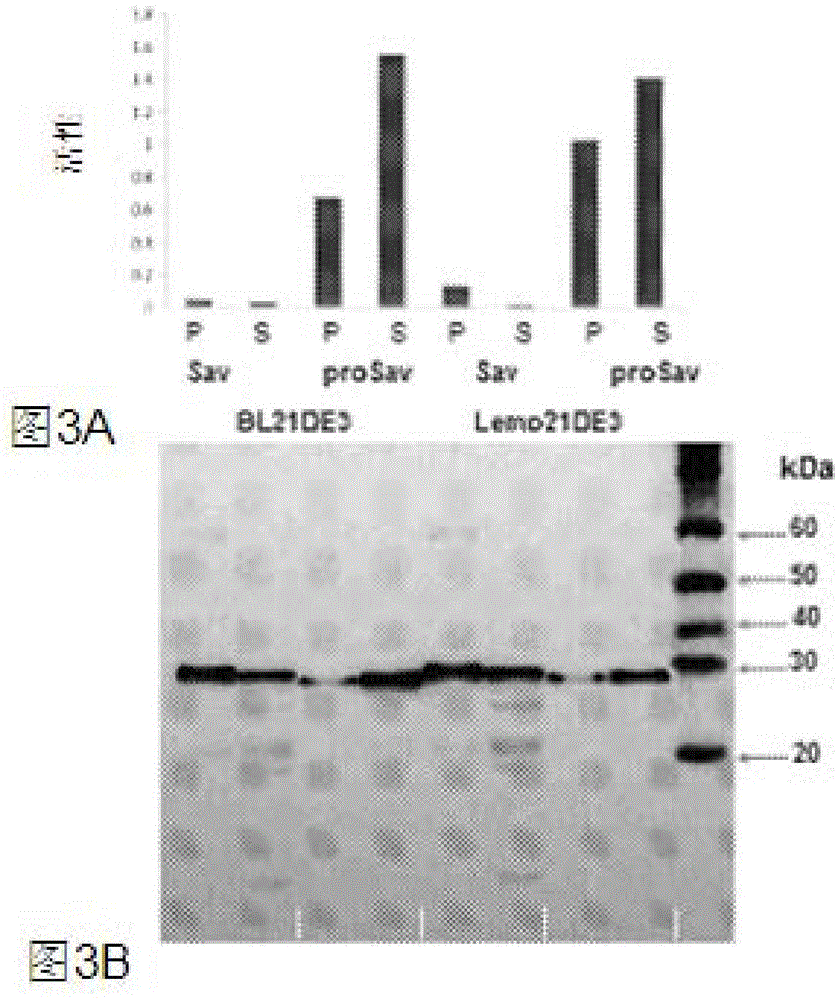
[0171] For the expression of keratinase for use in animal feed, the following steps were carried out: Bacillus licheniformis PWD-1 keratinase (Q53521 ) can be expressed as a pro-enzyme in the endosperm or embryo of maize seeds. The expression cassette was optimized, different optimized codon versions of the coding sequence were tested, and the promoter, 5'-UTR intron and targeting signals were evaluated. Fertility, set and seed viability were tested. Multiple T1 seeds from a single event were screened to identify high expressers. The molecular mass, accumulation level and activity were detected, and the specific activity, MW, pH and optimum temperature of seed expressed keratinase were compared with those of enzymes produced by microorganisms. Seeds expressing keratinases can be evaluated in feeding experiments as a surrogate for microbial keratinases. Inoculation of keratinase into elite inbred germplasm can be used as the start of...
Embodiment 2
[0172] Example 2 Expression of Q53521 Keratinase Precursor Enzyme in Maize Seeds
[0173] A maize codon-optimized gene for Q53521 keratinase from B. licheniformis (gi 998767) was synthesized (SEQ ID NO: 12). The codon optimized gene of Q53521 was cloned into pUC57 to construct pUC57:FPROT Q53. To facilitate cloning between pBluescript and the EcoRI and XhoI sites of Uni ZAP XR (Agilent) of bacteriophage lambda, the XhoI site at bases 898-903 was removed by silent mutation C900 to G (on the tagged sequence) using site-directed mutagenesis point(CTCGAG). The plant expression constructs carried the complete XhoI site.
[0174] Nucleotide and protein sequences of the maize codon-optimized Q53521 keratinase from Bacillus licheniformis:
[0175]
[0176] The amino acid sequence and domain structure of the Q53521 keratinase from Bacillus licheniformis PWD-1 (http: / / www.uniprot.org) shown below includes the amino terminus between amino acid residues 1-29 (underlined) Signal pep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com