Application of schisandrin B in preparation of drugs used for preventing colitis or colorectal carcinomas
A technology of Schisandra B and colitis, which is applied in the directions of drug combination, antitumor drug, active ingredient of heterocyclic compounds, etc., can solve the problems such as no literature report and patent disclosure, and achieves the reduction of the production of inflammatory factors. Prospects for application and good druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
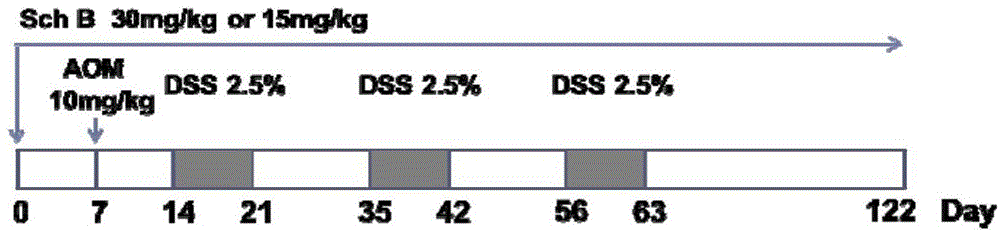
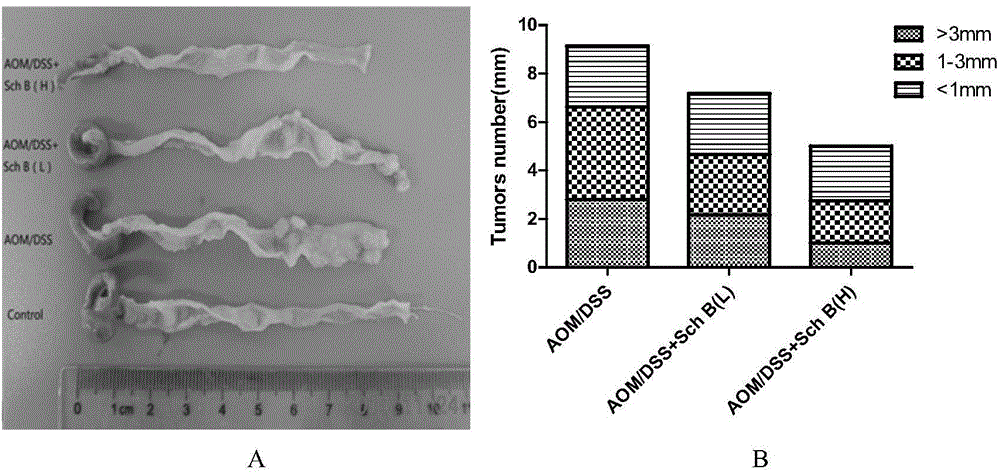
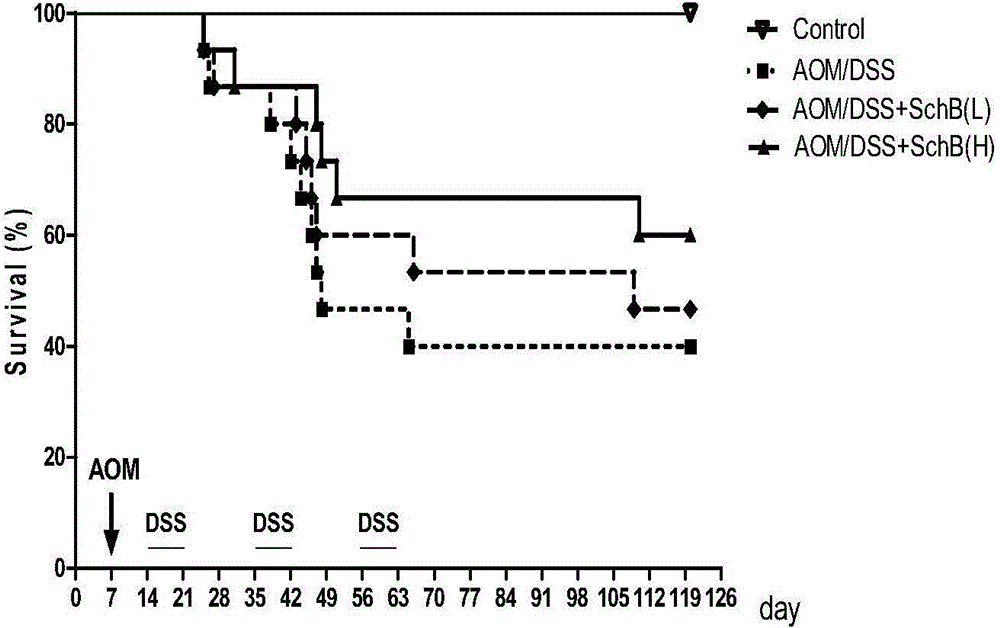
[0036] Example 1: Schizandrin B reduces the occurrence of colonic tumors associated with colitis
[0037] (1) Experimental materials:
[0038] Experimental reagent: Schizandrin B (purity ≥ 95%, here is the mass percentage), with 5mg / ml CMC-Na solution respectively made into two concentrations of 1.5mg / ml and 0.75mg / ml, carried out by 20ml / kg mouse body weight Administration; Carboxymethylcellulose sodium (CMC-Na) was purchased from Sinopharm Group, and was made into a 5mg / ml solution with double distilled water; azoxymethane (AOM) was purchased from Sigma Company, and was made into a 0.5mg / ml solution with normal saline The AOM working solution of ml was administered according to 20 ml / kg mouse body weight; dextran sodium sulfate (DSS) was purchased from MP Biomedicals, and a 25 mg / ml DSS solution was prepared with physiological saline.
[0039] Experimental animals: C57 male mice, 8 weeks old, weighing 18-20 g, purchased from Beijing Weitong Lihua.
[0040] (2) Experimental...
Embodiment 2
[0056] Example 2: Schizandrin B reduces inflammatory conditions in colitis-associated colon cancer
[0057] (1) Experimental materials:
[0058] Experimental reagents: mTNF-α (EM008), mIL-1β (EM001), mIL-6 (EM004), mIL-10 (EM005), mTGF-β1 (EM010) ELISA kits were purchased from Shanghai Yikesai Biological Company; Primers for mTNF-α, mIL-1β, mIL-6, mIL-10, mTGF-β1 were set at GenScript, Nanjing; Takara, PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time), Code No.RR047A; Takara, Premix Ex Taq TM II (Tli RNaseH Plus), Code No.RR820A; BCA protein quantification kit.
[0059] Experimental equipment: Nucleic acid quantitative instrument (Eppendorf), constant temperature water bath, ordinary PCR instrument, ABI Step One Plus real-time quantitative PCR instrument, automatic microplate reader, desktop high-speed refrigerated centrifuge, biochemical incubator.
[0060] (2) Experimental method:
[0061] 1) Blood was collected from the orbital venous plexus of the AOM / ...
Embodiment 3
[0095] Example 3: Schizandrin B reduces the occurrence of colitis
[0096] (1) Experimental materials and experimental equipment are the same as in Example 1.
[0097] (2) Experimental method
[0098] Thirty-two C57 male mice were randomly divided into 4 groups, 8 in each group: solvent control group, DSS model group, DSS+Sch B high-dose group, DSS+Sch B low-dose group. Solvent control group: in the first week of the experiment, the mice were given 5 mg / ml CMC-Na solution by intragastric administration every day, and began to drink water freely for 8 days on the 8th day; DSS model group: in the first week of the experiment, the mice were given 5 mg / ml by intragastric administration every day CMC-Na solution, given 25mg / ml DSS solution on the 8th day, and then freely drinking water for 8 days; DSS+Sch B high / low dose group: In the first week of the experiment, mice were given Sch B (30mg / kg or 15mg / kg mouse body weight) solution, on the 8th day, each group began to give 25mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com