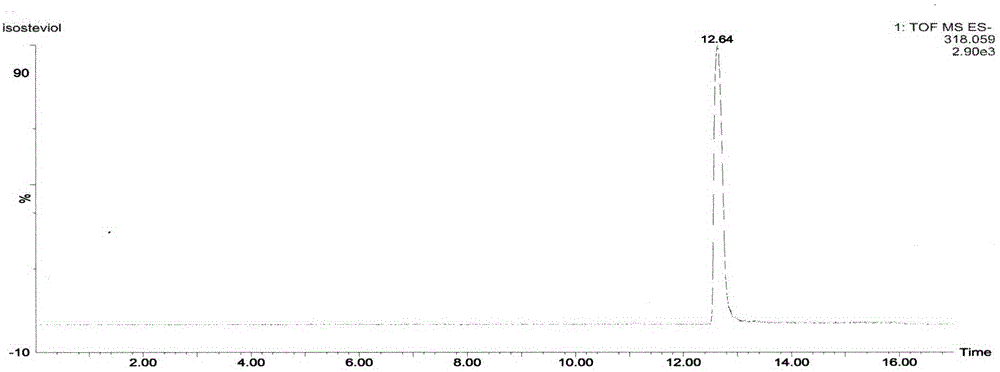
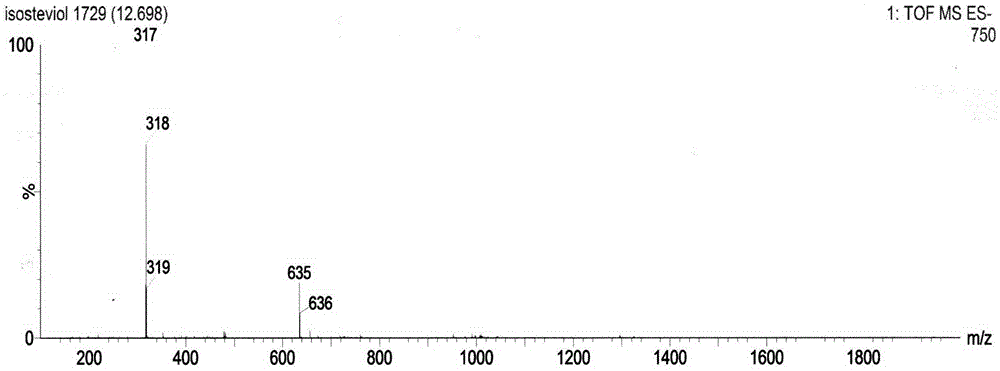
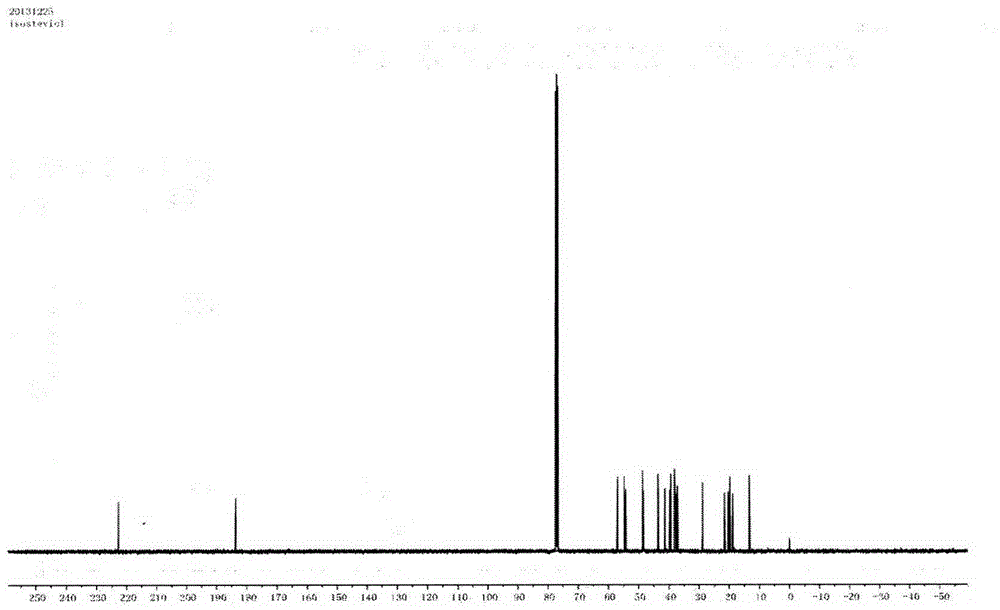
Method for preparing isosteviol through catalytic hydrolysis of stevioside by chlorinated aromatic sulfonic resin
A technology of chloroarylsulfonic acid and steveside, which is applied in the preparation of carboxylates, organic compounds, organic chemistry, etc., can solve the problems of polluting the environment, corroding equipment, high requirements for enzyme selection, and high cost. achieve the effect of speeding up the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] With 540mL concentration of 100g / L of stevioside solution (the purity of stevioside is 95%, containing RA4.0%, RC0.2%, others are all compounds with steviol glycoside characteristic ultraviolet absorption, solvent is 5% DMF aqueous solution) was preheated at 90°C for 0.5 hour; then the preheated solution was passed through a packed column equipped with a chloroarenesulfonic acid type ion-exchange macroporous resin catalyst at a flow rate of 5mL / h, and the volume of the packed column was 50mL , the resin exchange capacity is 4.7mmol / g, and the jacket of the packed column is heated with external circulating water so as to control the reaction temperature at 90°C. The effluent then enters a storage tank with a temperature of 5° C., and 5 mL of 5% DMF aqueous solution is placed in the storage tank. The product isosteviol is precipitated in a low-temperature storage tank. The effluent is preheated at 90° C. The flow rate of h is circulated into the catalyst column, and after...
Embodiment 2
[0040] 500mL of steviol glycoside solution with a concentration of 150g / L (the purity of stevioside is 80%, containing 15% of RA, 2% of RC, and the others are all compounds with steviol glycoside characteristic ultraviolet absorption, and the solvent is water) at 80°C Preheating for 0.5 hour; then this preheating solution is passed through a packed column equipped with a chlorinated arylsulfonic acid type ion exchange macroporous resin catalyst at a flow rate of 2mL / h, the volume of the packed column is 50mL, and the resin exchange capacity is 4.96mmol / g, the packed column jacket is heated with external circulating water so that its reaction temperature is controlled at 80°C; the effluent then enters a storage tank with a temperature of 25°C, and 5mL of water is placed in the storage tank, and the product isosteviol is stored in a low-temperature storage tank Precipitated, the effluent was preheated at 80°C for 0.5 hours and then circulated into the catalyst column. After 10 h...
Embodiment 3
[0042] 700mL concentration of 50g / L of stevioside solution (the purity of stevioside is 98%, containing 1.5% of RA, and others are all compounds with steviol glycoside characteristic ultraviolet absorption, and the solvent is 10% DMF aqueous solution) in Preheat at 95°C for 0.5 hour; then pass this preheated solution through a packed column equipped with a chloroarenesulfonic acid type ion-exchange macroporous resin catalyst at a flow rate of 2mL / h, the volume of the packed column is 50mL, and the resin exchange capacity is 4.96 mmol / g, the packed column jacket is heated with external circulating water so that its reaction temperature is controlled at 95°C; the effluent enters a storage tank with a temperature of 0°C, and 5mL of 10% DMF aqueous solution is placed in the storage tank, and the product isosteviol Precipitated in a low-temperature storage tank, the effluent was preheated at 95°C for 0.5 hours and then circulated into the catalyst column. After 6 hours of reaction, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| crystallization temperature | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com