Method for synthesizing 1,5-diaminonaphthalene by one step with naphthalene
A technology of diaminonaphthalene and mixed solvents, applied in the preparation of amino groups replacing hydrogen atoms, organic chemistry, etc., can solve problems such as environmental pollution and complex processes, and achieve the effects of short process, simple process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
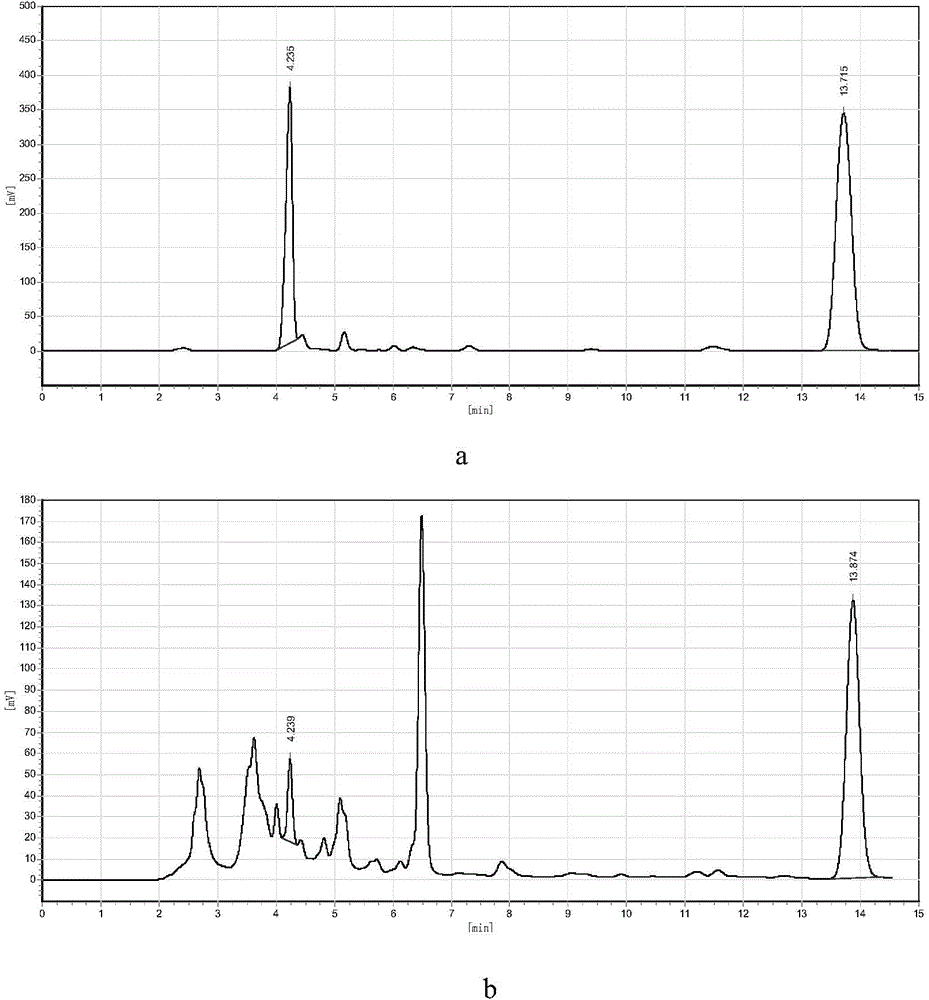
[0028] Weigh 0.05g of sodium metavanadate and 1.6g of hydroxylamine hydrochloride respectively, put them into a three-necked flask connected with a thermometer and a condenser tube, then measure 15mL of glacial acetic acid and 5mL of deionized water into the three-necked flask, and then place the three-necked flask In a constant temperature water bath, stir at 30°C for 20 minutes, then add 0.5 g of naphthalene, raise the temperature to 80°C, and stop the reaction after 4 hours of constant temperature reaction. The final product was obtained after post-processing the reaction liquid, and the mass of 1,5-diaminonaphthalene in the product was quantitatively analyzed by liquid chromatography external standard method, and the yield was 2.3%.
[0029] From figure 1 It can be seen from the figure that the reaction produces 1,5-diaminonaphthalene, wherein the retention time of 1,5-diaminonaphthalene is about 4.2 minutes, and the retention time of raw naphthalene is about 13.8 minutes....
Embodiment 2
[0031] Weigh 0.05g of sodium metavanadate and 1.6g of hydroxylamine hydrochloride respectively, put them into a three-necked flask connected with a thermometer and a condenser tube, then measure 20mL of glacial acetic acid and 5mL of deionized water into the three-necked flask, and then place the three-necked flask In a constant temperature water bath, stir at a constant temperature of 30°C for 20 minutes, then add 0.5g of naphthalene, raise the temperature to 80°C, and stop the reaction after 6 hours of constant temperature reaction. The final product was obtained after post-treatment of the reaction liquid, and the mass of 1,5-diaminonaphthalene in the product was quantitatively analyzed by liquid chromatography external standard method, which was 31.8 mg, and the yield was 5.15%.
Embodiment 3
[0033] Weigh 0.05g of sodium metavanadate and 1.6g of hydroxylamine hydrochloride respectively, put them into a three-necked flask connected with a thermometer and a condenser tube, then measure 20mL of glacial acetic acid and 5mL of deionized water into the three-necked flask, and then place the three-necked flask In a constant temperature water bath, stir at a constant temperature of 30°C for 20 minutes, then add 0.5g of naphthalene, raise the temperature to 60°C, and stop the reaction after 8 hours of constant temperature reaction. The final product was obtained after post-treatment of the reaction liquid, and the mass of 1,5-diaminonaphthalene in the product was quantitatively analyzed by liquid chromatography external standard method, which was 6.8 mg, and the yield was 1.1%.
[0034] This embodiment illustrates that the conversion rate of naphthalene is relatively low when the temperature is lower, and an appropriate temperature range is more conducive to improving the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com