Method for producing precipitated barium sulfate and co-producing manganese chloride
A technology for precipitating barium sulfate and manganese chloride, which is applied in the direction of manganese halide, calcium/strontium/barium sulfate, etc., can solve the problems of low added value and economic benefits of products, high impurity content of products, complicated process flow, etc., and achieve cost Low cost, reduced product cost, rich raw material resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
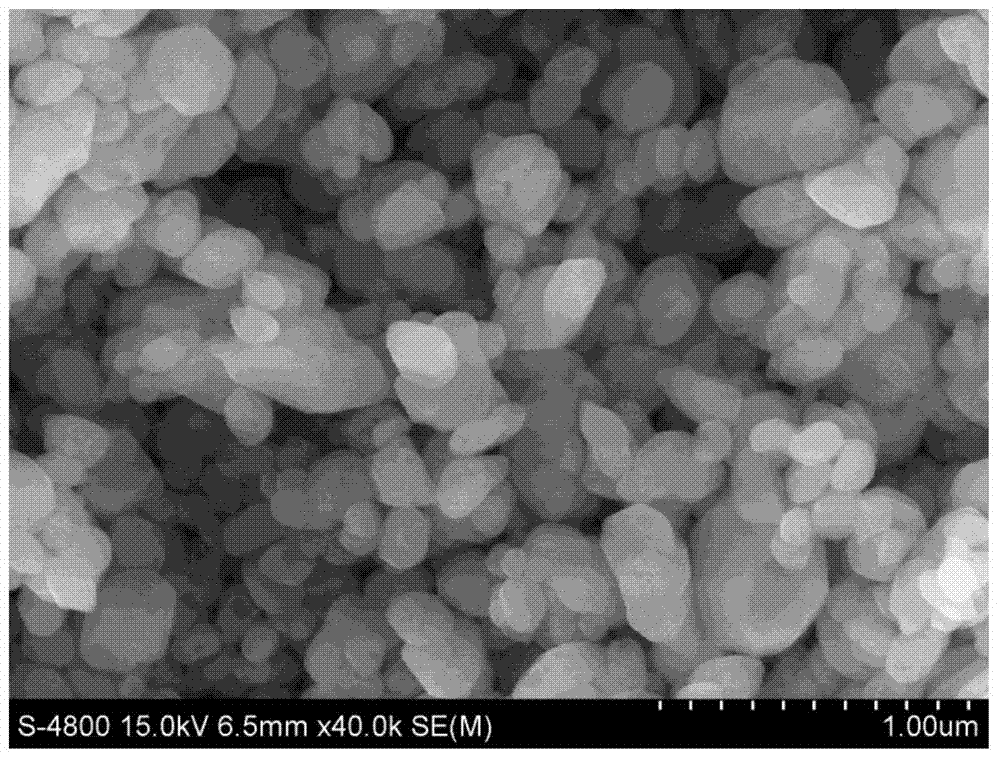
Examples
Embodiment 1
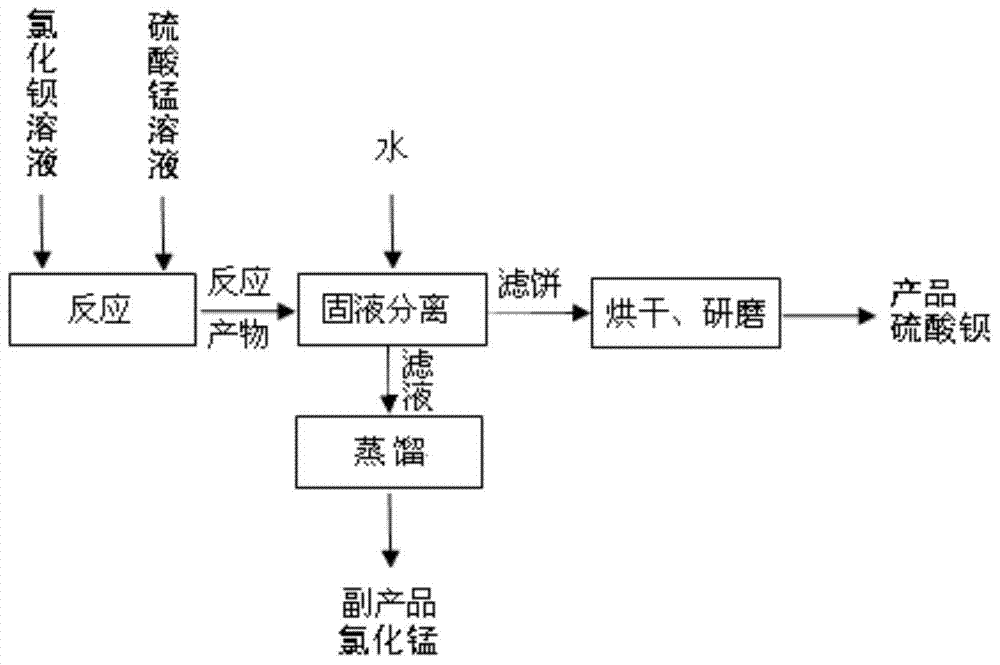
[0043] A. Prepare barium chloride solution: dissolve solid barium chloride in water, prepare barium chloride solution with a concentration of 0.6mol / L, take out 500mL, add 0.83g of P5040, stir evenly, and set aside;
[0044] B. Prepare manganese sulfate solution: dissolve solid manganese sulfate in water, prepare manganese sulfate solution with a concentration of 0.6mol / L, take out 500mL, adjust the pH to 2.5 with sulfuric acid or hydrochloric acid, and set aside;
[0045] C, metathesis reaction: the manganese sulfate solution prepared by step B is stirred and dripped into the barium chloride solution prepared by step A, and the excessive 3% of manganese sulfate solution is controlled, and the stirring speed is controlled to be 200r / min. Carry out metathesis reaction under;
[0046] D, filter the product after step C metathesis reaction, collect filtrate and filter cake respectively;
[0047] E, carry out SO with the filtrate collected in step D 4 2- Removal of impurities, ...
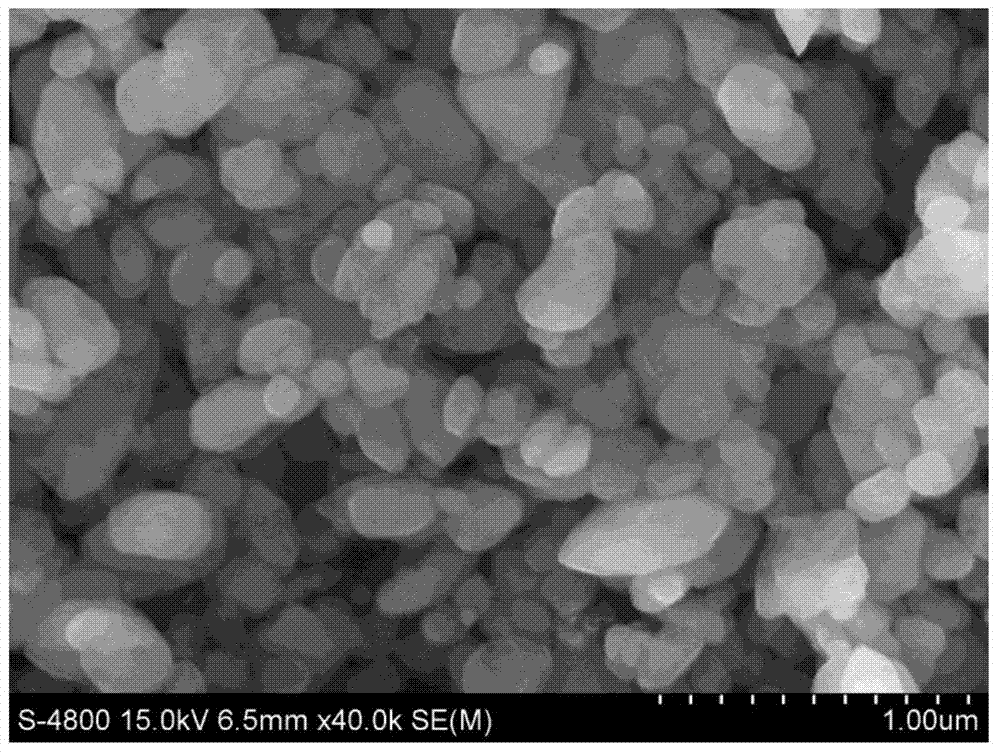
Embodiment 2
[0050] A. Prepare barium chloride solution: dissolve solid barium chloride in water, prepare barium chloride solution with a concentration of 0.1mol / L, take out 500mL, add 0.14g of P5040, stir evenly, and set aside;
[0051] B. Prepare manganese sulfate solution: dissolve solid manganese sulfate in water, prepare a manganese sulfate solution with a concentration of 0.1mol / L, take out 500mL, adjust the pH to 1 with hydrochloric acid, and set aside;
[0052] C, metathesis reaction: the manganese sulfate solution prepared by step B is stirred and dripped into the barium chloride solution prepared by step A, and the excessive 3% of manganese sulfate solution is controlled, and the stirring speed is controlled to be 200r / min. Carry out metathesis reaction under;
[0053] D, filter the product after step C metathesis reaction, collect filtrate and filter cake respectively;
[0054] E, carry out SO with the filtrate collected in step D 4 2- Removal of impurities, specifically: the...
Embodiment 3
[0057] A. Prepare barium chloride solution: dissolve solid barium chloride in water, prepare barium chloride solution with a concentration of 0.5mol / L, take out 500mL, add 4.16g of P5040, stir evenly, and set aside;
[0058] B. Prepare manganese sulfate solution: dissolve solid manganese sulfate in water, configure manganese sulfate solution with a concentration of 0.5mol / L, take out 500mL, adjust the pH to 1.5 with hydrochloric acid, and set aside;
[0059] C, metathesis reaction: the manganese sulfate solution prepared by step B is stirred and dripped into the barium chloride solution prepared by step A, and the excessive 3% of manganese sulfate solution is controlled, and the stirring speed is controlled to be 200r / min. Carry out metathesis reaction under;
[0060] D, filter the product after step C metathesis reaction, collect filtrate and filter cake respectively;
[0061] E, carry out SO with the filtrate collected in step D 4 2- Impurity removal is specifically: the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com