Method for synthesizing linaclotide
A technology of linaclotide and crude peptide, applied in the field of synthesizing linaclotide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of Fmoc-Tyr(tBu)-2-Cl Trt-resin
[0052] Take 500 g of 2-Cl Trt-Cl resin with a substitution value of 0.6 mmol / g, and add DMF to swell the resin. Take 0.6mol Fmoc-Tyr(tBu)-OH, dissolve it with an appropriate amount of DMF, add it to the above resin, stir well, then add 1.2mol DIPEA, stir for 3 hours, remove the reaction solution, wash with DMF for 3 times, then wash with DCM 3 times to obtain Fmoc-Tyr(tBu)-2-Cl Trt-resin with a substitution value of 0.45 mmol / g.
Embodiment 2
[0053] Embodiment 2: the synthesis of Fmoc-Tyr (tBu)-Wang resin
[0054] Take 500 g of Wang resin with a substitution value of 0.5 mmol / g, and add DMF to swell the resin. Take 0.5mol Fmoc-Tyr(tBu)-OH, dissolve it with an appropriate amount of DMF, add it to the above resin, stir well, then add 1.0mol DIC, 0.4molHOBt, 0.04mol 4-N,N-lutidine, and stir to react After 6 hours, the reaction solution was removed, washed three times with DMF and three times with DCM to obtain Fmoc-Tyr(tBu)-Wang resin with a substitution value of 0.39 mmol / g.
Embodiment 3
[0055] Embodiment 3: Preparation of linaclotide resin
[0056] Take 0.1mol of the Fmoc-Tyr(tBu)-2-Cl Trt-resin of Example 1 (substitution value 0.45mmol / g), deprotect it with 20% PIP / DMF solution for 25 minutes, wash and filter to obtain the H- Tyr(tBu)-2-Cl Trt-resin.
[0057] Take 0.3mol Fmoc-Cys(Trt)-OH and 0.3mol HOBt, and dissolve them with an appropriate amount of DMF; take another 0.3mol DIC, add slowly under stirring, continue stirring for 30 minutes, and add to the above H-Tyr(tBu)-2 -Cl Trt-resin, coupling reaction 120 ~ 300 minutes, the reaction end point is determined by the ninhydrin method, wash and filter, then deprotect with 20% PIP / DMF solution for 25 minutes, wash and filter to get H-Cys ( Trt)-Tyr(tBu)-2-Cl Trt-resin.
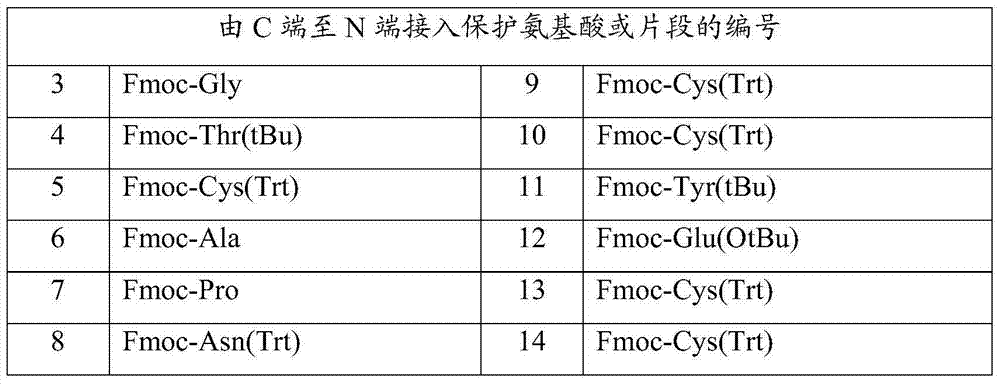
[0058] In the same way as above, the remaining protected amino acids in Table 3 were sequentially inserted to obtain the linaclotide peptide resin:
[0059] H-Cys(Trt)-Cys(Trt)-Glu(OtBu)-Tyr(tBu)-Cys(Trt)-Cys(Trt)-Asn(Trt)-Pro-Ala-Cys(Trt)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com