Drug releasing coating for medical device
A technology of medical equipment and coating, applied in the field of drug release coating for medical equipment, which can solve problems such as easy hydrolysis or oxidation, sensitivity to moisture, and complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0285] Preparation of coating solution (if necessary, add not more than 10% by volume of a small amount of water sufficient to dissolve all solutes):
[0286] Formulation 1.1 - 30-90mg rapamycin, 1-2% (based on the weight of rapamycin) butylated hydroxytoluene (BHT), 15-90mg Tween 80, 30-90mg docusate sodium (sulfonate Sodium diethylhexyl succinate) and 1-3ml ethanol were mixed.
[0287] Formulation 1.2 - Mix 30-90mg rapamycin, 1-2% (based on weight of rapamycin) butylated hydroxyanisole (BHA), 15-90mg Tween 80, 30-90mg docusate sodium (Sodium diethylhexyl sulfosuccinate) and 1-3ml ethanol were mixed.
[0288] Formulation 1.3 - Mix 30-90 mg rapamycin without added BHT or BHA, 15-90 mg Tween 80, 30-90 mg docusate sodium (diethylhexyl sodium sulfosuccinate) and 1-3 ml ethanol .
[0289] Formulation 1.4 - Mix 30-90mg rapamycin, 1-2% (based on the weight of rapamycin) BHA or BHT, 15-90mg Solutol HS 15, 5-30mg sodium lauryl sulfate and 1-3ml ethanol mix.
[0290] Formulation 1...
Embodiment B
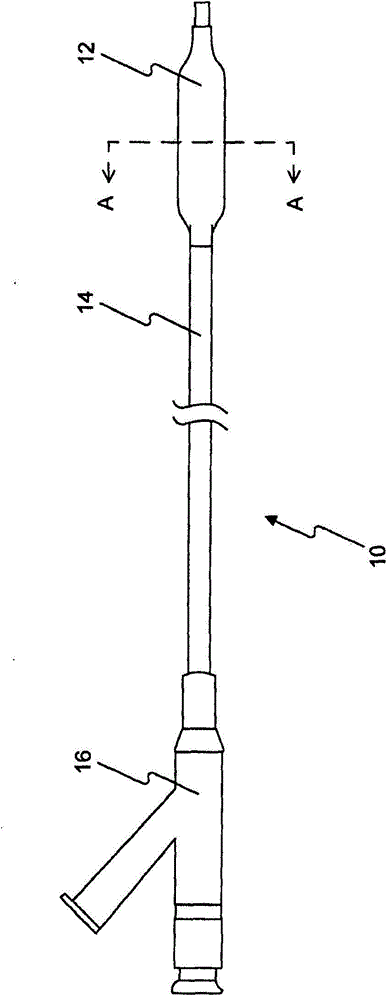
[0312] Five PTCA balloon catheters (3 mm in diameter and 20 mm in length) were coated using the method described in US Patent Application Publication No. 2010-0055294-A1 , which is hereby incorporated by reference in its entirety. Inflate the PTCA balloon catheter at 1-3 atmospheres. The inflated balloons were loaded, sprayed or dipped into the formulations of Example A (1.1-1.3). Then, the balloons were dried, drug loaded, sprayed or dipped again until balloons with sufficient amount of drug (3 μg / mm) were obtained. The coated balloons are folded, then repackaged and sterilized for analytical testing. The recovered rapamycin content was 79% for Formulation 1.1 with BHT; 100% for Formulation 1.2 with BHA; and 14%-50% for Formulation 1.3 without BHT or BHA. Antioxidants (BHT or BHA) prevent rapamycin from being oxidized or degraded.
Embodiment C
[0314] Six PTCA balloon catheters (3.5 and 3.0 mm in diameter and 20 mm in length) were inflated at 1-3 atmospheres. The inflated balloons were loaded with formulations 1.1-1.25 in Example A. A sufficient amount of drug (3-4 μg / mm2) was obtained on the balloon. The inflated balloon is folded and allowed to dry. The coated folded balloon is then repackaged, sterilized, and optionally vacuum dried for animal testing.
[0315] step : Insertion of coated PTCA balloon catheters into target sites (LAD, LCX and RCA) in the coronary vessels of 25-45 lb pigs. The balloon is inflated to about 12 atmospheres of pressure. The overdraw ratio (the ratio of balloon diameter to tube diameter) is about 1.15-1.40. During 30-60 seconds of inflation, the drug is delivered to the target tissue. Then, the balloon catheter is deflated and recovered from the animal. Target vessels were harvested 0.25-24 hours after the procedure. The drug content in the target tissue and the residual drug re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com