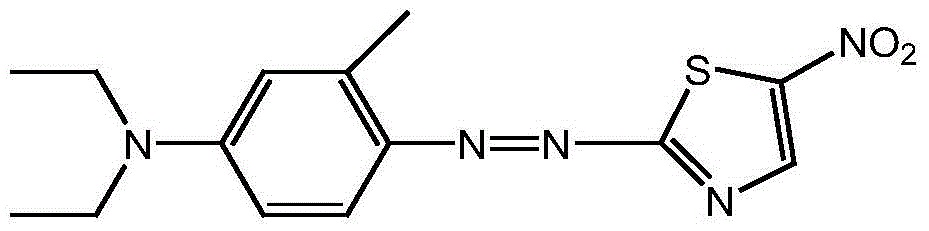
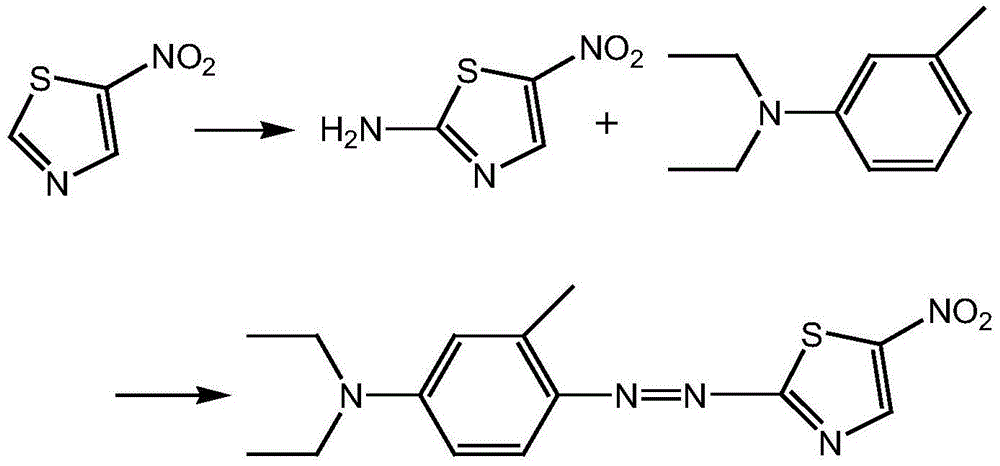
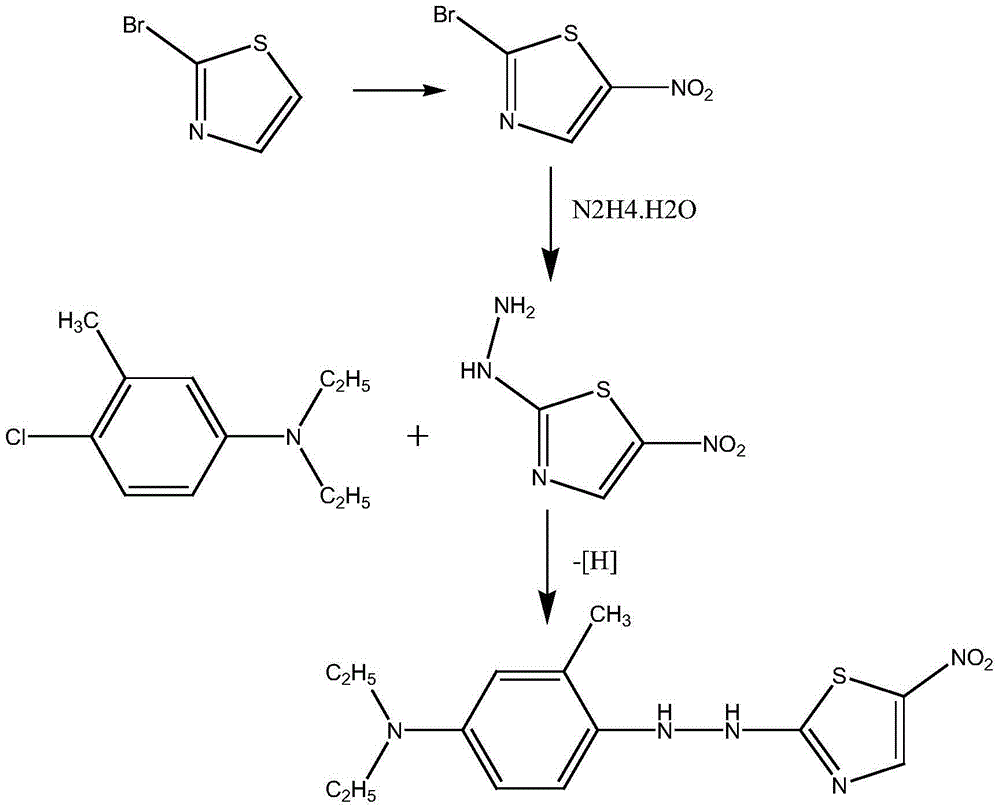
Method used for preparing disperse blue 360
A technology of disperse blue and nitrothiazole, which is applied in the synthesis of disperse blue 360, known in the field of preparation of disperse dyes, can solve the problems of difficult reaction, low product purity, and complicated impurities, so as to achieve a clean reaction system and avoid Acidic wastewater, the effect of good development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of step 1,2-bromo-5-nitrothiazole
[0036] Add 50g of concentrated sulfuric acid and 50g of concentrated nitric acid into a 250ml four-necked bottle with mechanical stirring and a thermometer, cool down to 15°C, then add 25g of 2-bromothiazole, stir and raise the temperature to 45°C and keep it warm for 2.5h; after the reaction, cool down to 20°C, add the reaction liquid to soft water, control the temperature below 20°C, filter, wash the filter cake with water until neutral, and dry to obtain 31.5g of solid product 2-bromo-5-nitrothiazole with a content of 97.5%. The rate is 96.0%.
Embodiment 2
[0038] The synthesis of step 1,2-bromo-5-nitrothiazole
[0039] Add 75g of concentrated sulfuric acid and 75g of concentrated nitric acid into a 250ml four-necked bottle with mechanical stirring and a thermometer, cool down to 15°C, then add 25g of 2-bromothiazole, stir and raise the temperature to 48°C and keep it warm for 2 hours; after the reaction, cool down to 20°C ℃, add the reaction solution to soft water, control the temperature below 20 ℃, filter, wash the filter cake with water until neutral, and dry to obtain 32.0 g of solid product 2-bromo-5-nitrothiazole, content 97.0%, yield 97%.
Embodiment 3
[0041] The synthesis of step 1,2-bromo-5-nitrothiazole
[0042] Add 100g of concentrated sulfuric acid and 100g of concentrated nitric acid into a 500ml four-necked bottle with mechanical stirring and a thermometer, cool down to 15°C, then add 25g of 2-bromothiazole, stir and raise the temperature to 55°C and keep it warm for 2h; after the reaction, cool down to 20°C ℃, add the reaction solution to soft water, control the temperature below 20 ℃, filter, wash the filter cake with water until neutral, and dry to obtain 31.2 g of solid product 2-bromo-5-nitrothiazole, content 98.0%, yield 95.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com