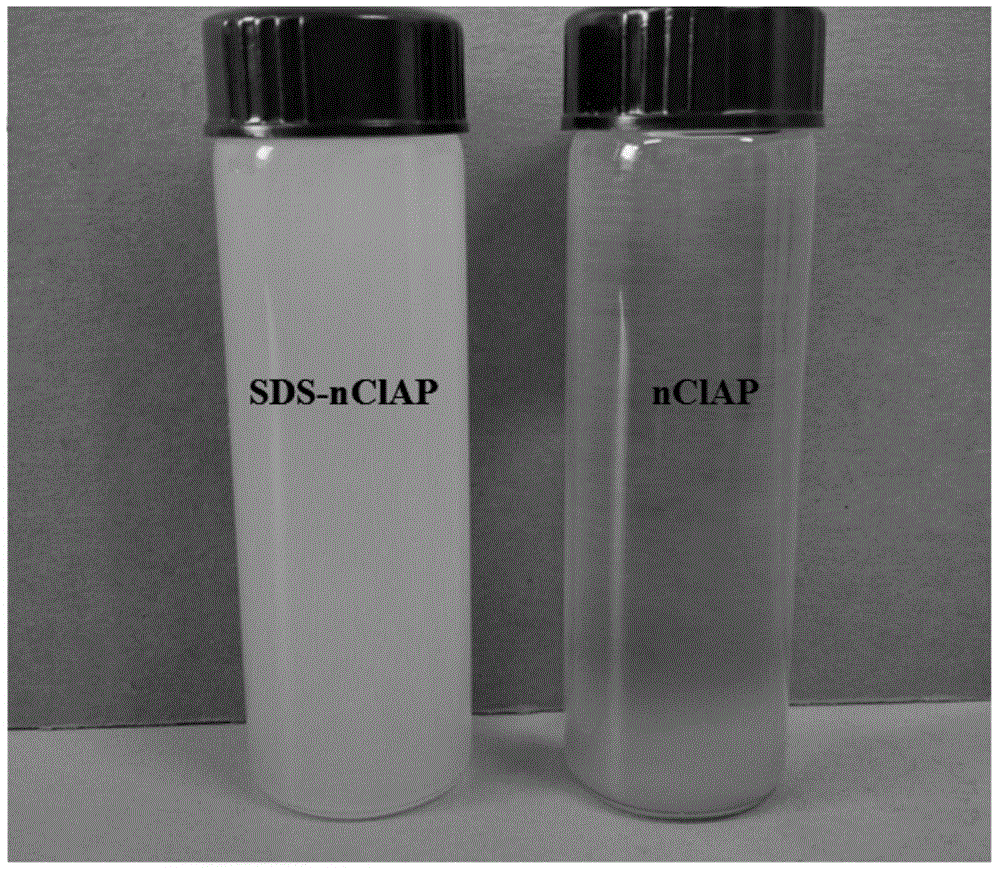Modified nanometer chlorapatite and preparation method thereof
A chloroapatite and nano-technology, applied in the field of modified nano-chloroapatite and its preparation, can solve the problems of difficult sediment migration, uneven dispersion, easy settlement, etc., and achieve beneficial sediment restoration and uniform dispersion , Improve the effect of reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
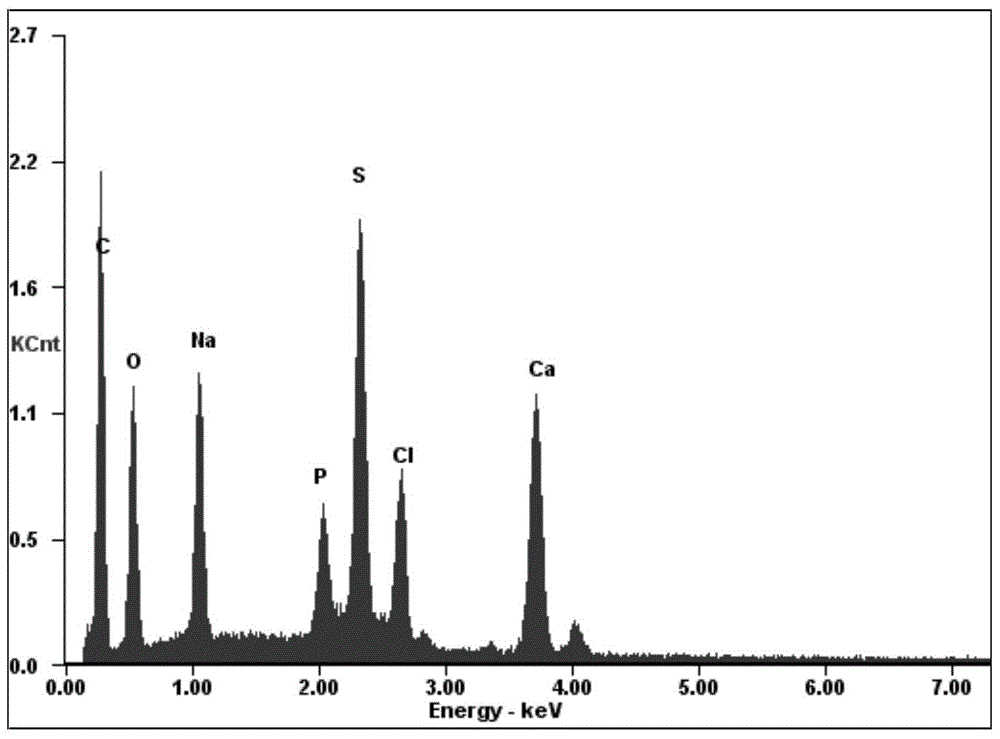
[0034] A modified nano chloroapatite (SDS-nClAP) of the present invention, the modified nano chloroapatite is composed of nano chloroapatite and sodium lauryl sulfate, sodium lauryl sulfate is modified on On the surface of nano chloroapatite, the mass ratio of sodium lauryl sulfate to nano chloroapatite is 7.1687:1. The particle size of the modified nano chloroapatite is 5nm-8nm.
[0035] A preparation method of the modified nano-chloroapatite of the above-mentioned present embodiment, comprising the following steps:
[0036] (1) CaCl 2 Solution preparation: weigh 1.9698g CaCl 2 2H 2 O into a beaker, add an appropriate amount of ultrapure water to dissolve and transfer to a 500mL volumetric flask, use ultrapure water to adjust the volume to the mark, and shake well to obtain 26.8mM CaCl 2 solution.
[0037] Na 3 PO 4 Solution preparation: weigh 3.04g Na 3 PO 4 12H 2 O into a beaker, add an appropriate amount of ultrapure water to dissolve and transfer to a 500mL volu...
Embodiment 2
[0045] A modified nano chloroapatite (SDS-nClAP) of the present invention, the modified nano chloroapatite is composed of nano chloroapatite and sodium lauryl sulfate, sodium lauryl sulfate is modified on On the surface of nano chloroapatite, the mass ratio of sodium lauryl sulfate to nano chloroapatite is 7.1687:1. The particle size of the modified nano chloroapatite is 5nm-8nm.
[0046] A preparation method of the modified nano-chloroapatite of the above-mentioned present embodiment, comprising the following steps:
[0047] (1) CaCl 2 Solution preparation: weigh 1.9698gCaCl 2 2H 2 O into a beaker, add an appropriate amount of ultrapure water to dissolve and transfer to a 500mL volumetric flask, use ultrapure water to adjust the volume to the mark, and shake well to obtain 26.8mM CaCl 2 solution.
[0048] Na 3 PO 4 Solution preparation: weigh 3.04g Na 3 PO 4 12H 2 O into a beaker, add an appropriate amount of ultrapure water to dissolve and transfer to a 500mL volum...
Embodiment 3
[0055] A modified nano chloroapatite (SDS-nClAP) of the present invention, the modified nano chloroapatite is composed of nano chloroapatite and sodium lauryl sulfate, sodium lauryl sulfate is modified on On the surface of nano chloroapatite, the mass ratio of sodium lauryl sulfate to nano chloroapatite is 14.3375:1. The particle size of the modified nano chloroapatite is 5nm-8nm.
[0056] A preparation method of the modified nano-chloroapatite of the above-mentioned present embodiment, comprising the following steps:
[0057] (1) CaCl 2 Solution preparation: weigh 1.9698gCaCl 2 2H 2 O into a beaker, add an appropriate amount of ultrapure water to dissolve and transfer to a 500mL volumetric flask, use ultrapure water to adjust the volume to the mark, and shake well to obtain 26.8mM CaCl 2 solution.
[0058] Na 3 PO 4 Solution preparation: weigh 3.04g Na 3 PO 4 12H 2 O into a beaker, add an appropriate amount of ultrapure water to dissolve and transfer to a 500mL volu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com