Arylmethylenemalononitrile-*Base solid-state luminescent material and method
A technology of aramethylenemalononitrile and luminescent materials, which is applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as fluorescence quenching, and achieve short reaction time, excellent solid luminescent performance, and high practicality. The effect of applying value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A kind of aryl methylene malononitrile- The preparation method of Base solid-state luminescent material comprises the following steps:
[0044] 1) Aldehyde substitution synthesis of aldehyde- Base compound, the specific method is:
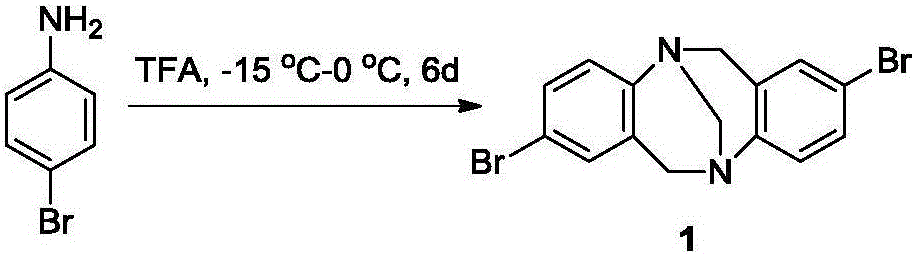
[0045] 1-1) Using 4-bromoaniline as raw material and paraformaldehyde under the catalysis of trifluoroacetic acid (TFA) at -15°C for 6 days to synthesize 2,8-dibromo Base, such as figure 1 shown;
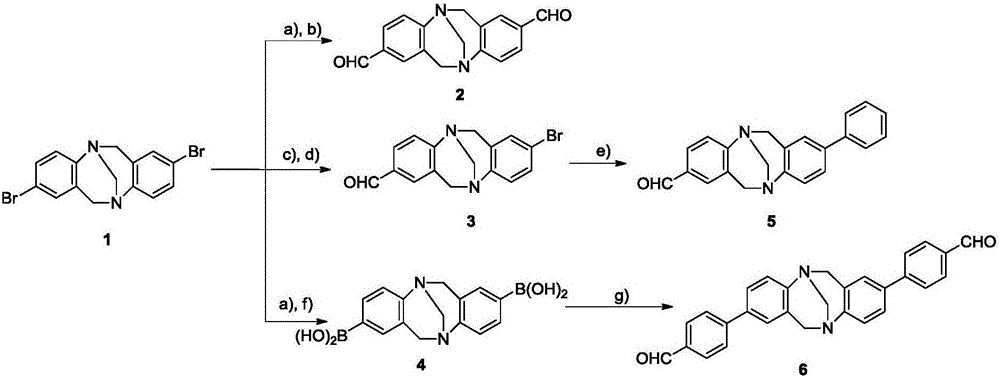
[0046] 1-2) 2,8-dibromo Base and aldehyde group are substituted to synthesize aldehyde group- Base compound, including three methods such as figure 2 shown; where the annotations in the figure are as follows:
[0047] a) n-BuLi, THF, -78°C, 1.5h;
[0048] b) DMF, -78℃-r.t., 2min;
[0049]c) n-BuLi,THF:Et 2 O=1:3, -78℃, 1.5h;
[0050] d) DMF, -78℃-r.t., 10min;
[0051] e) PhB(OH) 2 ,Pd(PPh 3 ) 4 ,2M K 2 CO 3 , toluene, 110°C;
[0052] f)(CH 3 O) 3 B, -78℃-r.t., 2min;
[0053] g) p-iodobenzaldehyde, Pd (PPh 3 ) 4 ,2M K ...
Embodiment 2
[0062] Example 2: Solid-state luminescent properties of the product
[0063] 1) UV absorption spectrum:
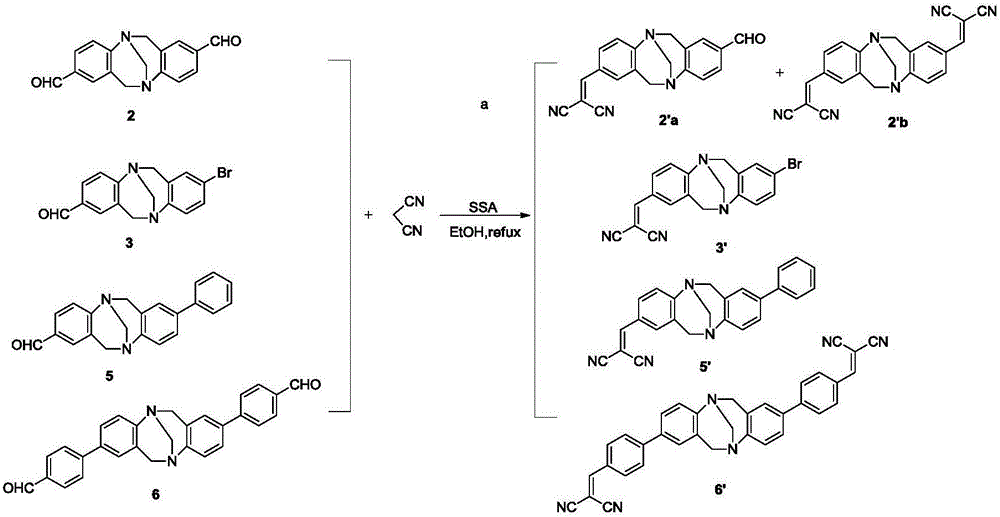
[0064] Aldehyde- Base compound 2, 3, 5, 6 and 2'a, 2'b, 3', 5', 6' make up 1×10 -6 mol / L dichloromethane solution, carry out ultraviolet absorption spectrum measurement, and corresponding ultraviolet absorption spectrum is as follows Figure 4 , 5 shown. Compounds 2, 3, and 6 have an obvious absorption band at 300-330nm, and compound 5 has absorption at 270-300nm, which is caused by the π-π* transition; compounds 2'a, 2'b, and 3 ', 5', 6' all have strong absorption at 350-400nm, which is caused by the π-π* transition; the absorption wavelengths of compounds 2'a, 2'b, 3', 5' and 6' are the same as those of compound 2 , 3, 5 and 6 have increased, which may be caused by the strong electron-withdrawing property of the cyano group.
[0065] 2) Solid state fluorescence:
[0066] The dichloromethane solution containing the above compound hardly shows fluorescence under th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com