Method for constructing curcuminoid SULTs metabolism finger-print and application
The technology of a metabolic fingerprint and a construction method is applied in the construction of the fingerprint of curcumin compound sulfonation binding reaction and the construction of the metabolic fingerprint of drug metabolizing enzymes, which can solve the difficulty in the development and application of new drugs of curcumin compounds, and the oral bioavailability. It can solve the problems of low degree and weak absorption of drug metabolism, etc., and achieve the effect of good ionization effect, good UV absorption characteristics and high sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
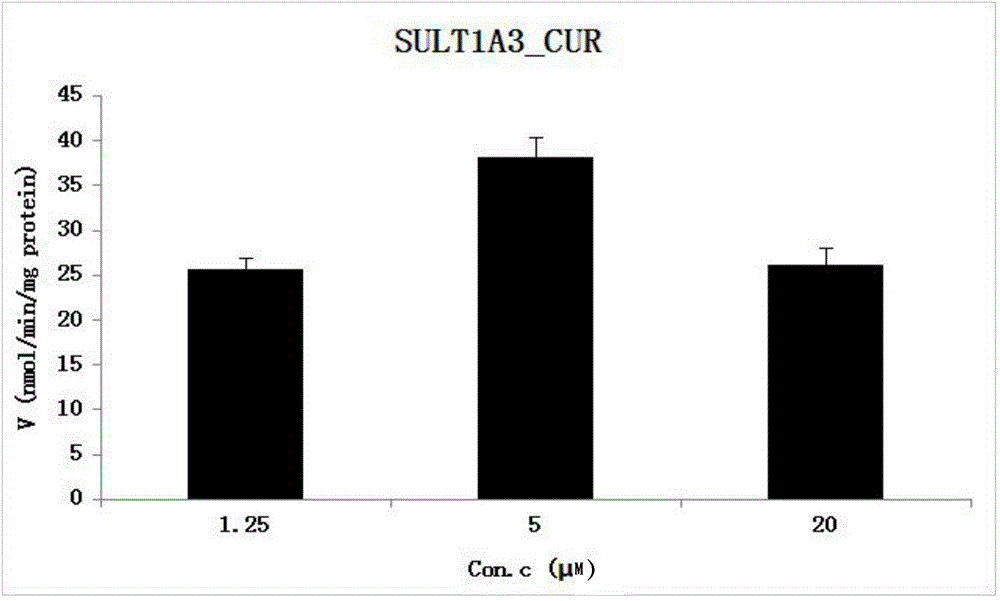
[0037] The reaction system of SULT1A3 catalyzing CUR sulfonation binding reaction, and the metabolic activity of SULT1A3 catalyzing CUR obtained by the method.
[0038] (1) Establishment of chromatographic quantitative analysis method for CUR and its sulfonated reaction metabolites: WATERS UPLC-HCLASS instrument; diode array detector PDA; ACQUITY BEH C 18 (1.7μm2.1×50mm) chromatographic column; mobile phase is 2.5mMpH6.5 ammonium acetate aqueous solution (A)-acetonitrile (B), gradient elution: 0min, 10%B; 0-2.0min, 10~40%B ;2.0-3.0min, 40~60%B; 3.0-3.5min, 60~70%B; 3.5-4.5min, 70~90%B; 4.5-5.0min, 90%B; 5.0-5.5min, 90%B ~10%B. The internal standard is estradiol; the flow rate is 0.25ml / min; the column temperature is 35°C; the detection wavelength of CUR is 430nm, and the internal standard detection wavelength is 280nm.
[0039] (2) Enzyme reaction: Prepare three different concentrations of CUR, namely 1.25 μM, 5 μM, and 20 μM. In the incubation reaction system, use pH 7.4 ...
Embodiment 2
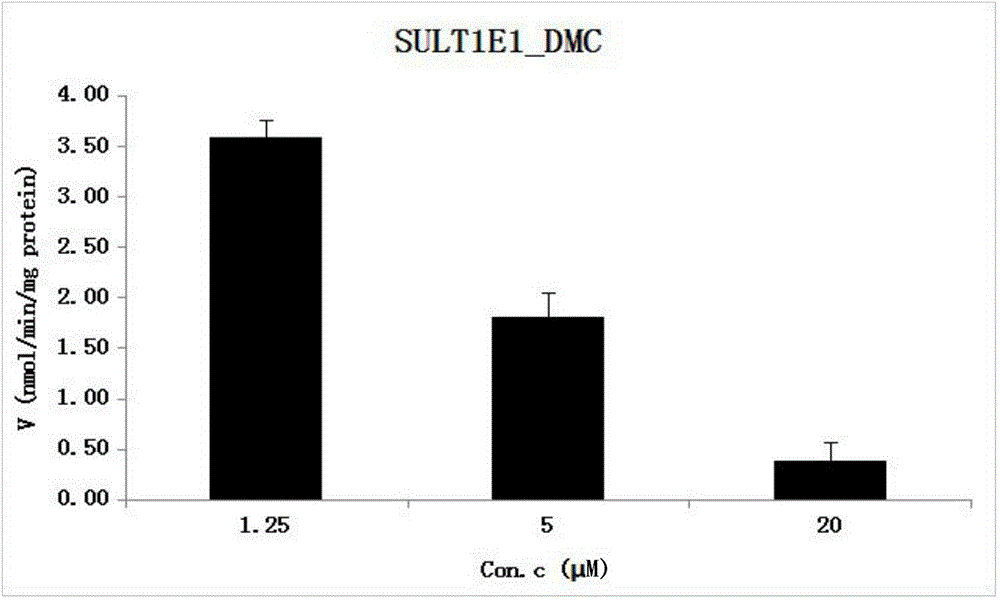
[0043] A reaction system in which SULT1E1 catalyzes the sulfonation reaction of DMC, and the metabolic activity of SULT1E1 catalyzed DMC obtained by the method.
[0044](1) Establishment of chromatographic quantitative analysis method for DMC and its sulfonated reaction metabolites: WATERS UPLC-HCLASS instrument; diode array detector PDA; ACQUITY BEH C18 (1.7μm2.1×50mm) chromatographic column; mobile phase is 2.5mM pH6.5 ammonium acetate aqueous solution (A)-acetonitrile (B), gradient elution is the same as CUR; internal standard is estradiol; flow rate is 0.25ml / min; the column temperature is 35°C; the DMC detection wavelength is 430nm, and the internal standard detection wavelength is 280nm.
[0045] (2) Enzyme reaction: This reaction system is similar to CUR: the three concentrations of DMC are 1.25 μM, 5 μM, and 20 μM, and SULT1E1 (final concentration is 0.00125-0.00248 mg / ml) is selected. Incubate in KPI buffer system, the reaction temperature is 37°C; the reaction tim...
Embodiment 3
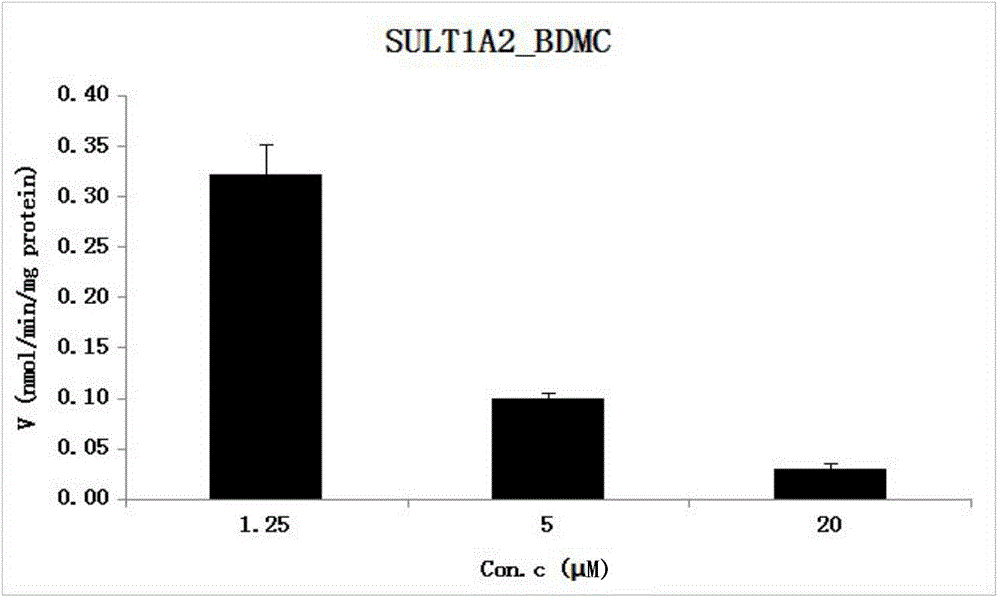
[0049] The reaction system of SULT1A2 catalyzing the sulfonation binding reaction of BDMC, and the metabolic activity of SULT1E1 catalyzing BDMC obtained by the method.
[0050] (1) Establishment of chromatographic quantitative analysis method for BDMC and its sulfonated reaction metabolites: WATERS UPLC-HCLASS instrument; diode array detector PDA; ACQUITY BEH C18 (1.7μm2.1×50mm) chromatographic column; mobile phase is 2.5mM pH6.5 ammonium acetate aqueous solution (A)-acetonitrile (B), gradient elution is the same as CUR; internal standard is estradiol; flow rate is 0.25ml / min; the column temperature is 35°C; the detection wavelength of BDMC is 430nm, and the detection wavelength of internal standard is 280nm.
[0051] (2) Enzyme reaction: The reaction system is similar to CUR: three concentrations of BDMC are 1.25 μM, 5 μM, 20 μM, SULT1A2 (final concentration is 0.0093 mg / ml), in KPI buffer liquid with cofactor PAPS at pH 8.5 Incubate in the system, the reaction temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com