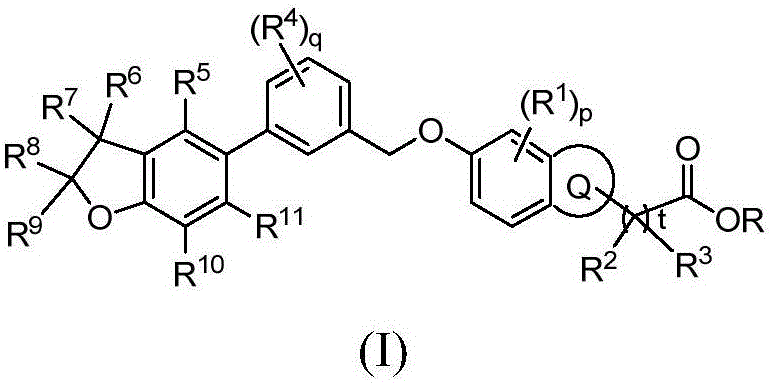
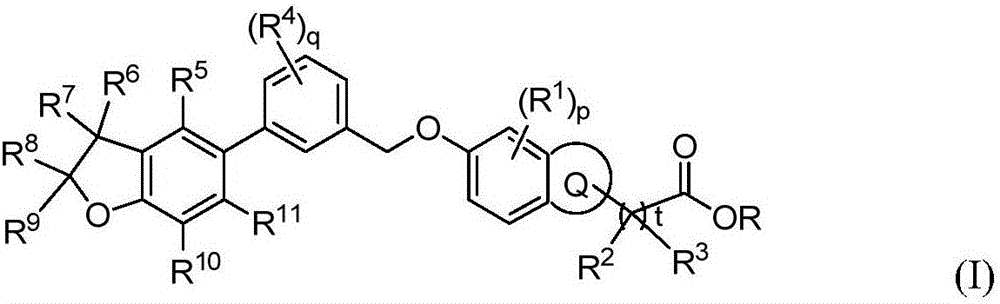
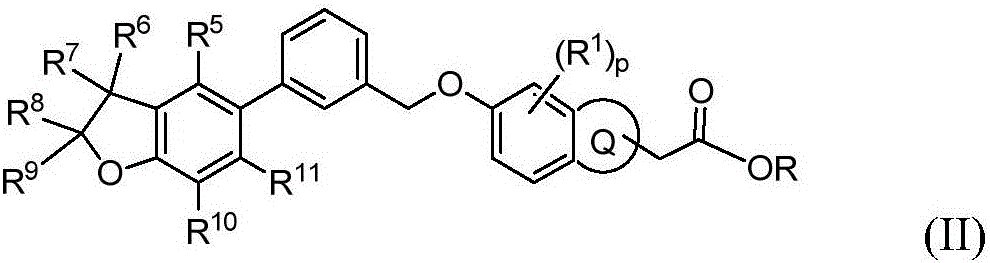
Benzofuran derivative, its preparation method and its application in medicine
A technology of pharmacy and compound, which is applied in the field of benzofuran derivatives, its preparation and its application in medicine, and can solve problems such as increasing the burden on diabetic patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0468] 2-(6-((3-(3-methoxy-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)-2 , 3-dihydrobenzofuran-3-yl)acetic acid (compound 1)
[0469] 2-(6-((3-(3-methoxy-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)-2,3-dihydrobenzofuran-3-yl)acetic acid
[0470]
[0471] The first step: 5-bromo-3-methoxy-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran (1a)
[0472] 5-bromo-3-methoxy-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran
[0473]
[0474] In an anhydrous and oxygen-free atmosphere, add sodium hydride (48 mg, 1.99 mmol) to 5-bromo-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran-3-ol under an ice-water bath 2G (450 mg, 1.66 mmol) in dry tetrahydrofuran (15 mL), stirred for 20 minutes. Iodomethane (471 mg, 3.32 mmol) was added to the mixture, and the mixture was raised to room temperature for 1 hour. After the reaction was completed, saturated aqueous ammonium chloride (15 mL) was added to quench the reaction, water (25 mL) was added, and extracted with ethyl aceta...
Embodiment 2
[0496] 2-(6-((3-(3-ethoxy-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)-2 , 3-dihydrobenzofuran-3-yl)acetic acid (compound 2)
[0497] 2-(6-((3-(3-ethoxy-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)-2,3-dihydrobenzofur an-3-yl) acetic acid
[0498]
[0499] The first step: 5-bromo-3-ethoxy-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran (2a)
[0500] 5-bromo-3-ethoxy-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran
[0501]
[0502] In an anhydrous and oxygen-free atmosphere, add sodium hydride (80 mg, 3.32 mmol) to 5-bromo-2,2,4,6-tetramethyl-2,3-dihydrobenzofuran-3-ol 2G under ice-cooling (750mg, 2.76mmol) in dry tetrahydrofuran (20mL) and stirred for 20 minutes. Ethyl iodide (0.45 mL, 3.52 mmol) was added to the mixture, raised to room temperature for 5 hours, heated to reflux for 2 hours, and the reaction was completed. Add water (20 mL) to dilute the reaction solution, add saturated aqueous ammonium chloride solution (10 mL), and extract with ethy...
Embodiment 3
[0524] 2-(6-((3-(3-(2-methoxyethyl)-4,6-dimethyl-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)- 2,3-Dihydrobenzofuran-3-yl)acetic acid (compound 3)
[0525] 2-(6-((3-(3-(2-methoxyethyl)-4,6-dimethyl-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)-2,3-dihydroben zofuran-3-yl) acetic acid
[0526]
[0527] The first step: 2-(5-bromo-4,6-dimethylbenzofuran-3-yl)acetonitrile (3a)
[0528] 2-(5-bromo-4,6-dimethylbenzofuran-3-yl)acetonitrile
[0529]
[0530] In an anhydrous and oxygen-free atmosphere, slowly add diethoxy cyanomethyl phosphate (1.1 g, 6.22 mmol) dropwise to a solution of sodium hydride (149 mg, 6.22 mmol) in tetrahydrofuran (20 mL) at 0°C, and stir for 10 minutes , 5-bromo-4,6-dimethylbenzofuran-3(2H)-one 2E (1.0g, 4.15mmol) tetrahydrofuran solution was slowly added dropwise to the mixture, raised to 35°C and continued to stir for 3 hours . Add saturated ammonium chloride solution (20mL) and water (20mL) to the reaction solution, extract with ethyl acetate (20mL×3), comb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com