Cefetamet pivoxil hydrochloride hydrate crystals and dispersible tablet thereof
A technology of ceftamet pivoxil hydrochloride and hydrate, which is applied in the field of ceftamet pivoxil hydrochloride hydrate crystals and its dispersible tablets, and can solve the problem of easy moisture absorption and deterioration of loading, variance, poor stability and fluidity of ceftamet pivoxil hydrochloride and other problems, to achieve the effect of good crystal stability, high bioavailability, and not easy to absorb moisture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
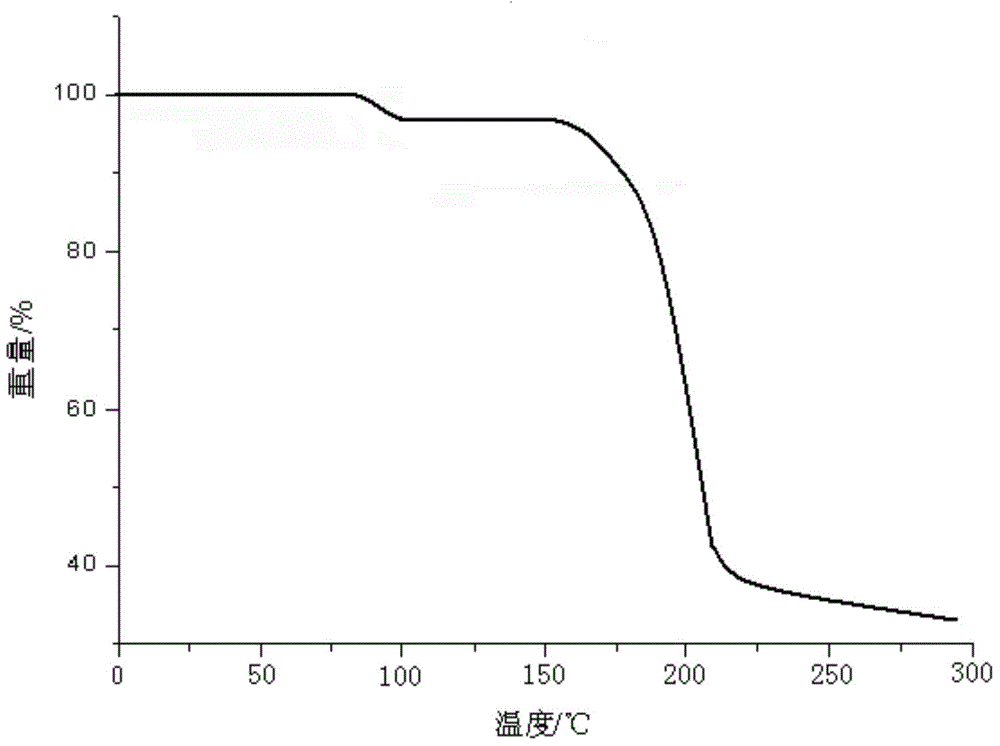
[0039] Get ceftazidime pivoxil hydrochloride crude product crude drug 10g and dissolve it in 30ml of ethanol at a temperature of 5°C, add ceftazidime pivoxil hydrochloride crude product quality 0.1 gac to decolorize for 20-30 minutes, filter, and wash with a small amount of ethanol solution to obtain ceftazidime hydrochloride Decolorize the ceftazidime pivoxil hydrochloride ethanol solution with activated carbon for 20 minutes, filter, and wash with a small amount of ethanol solution to obtain the ethanol solution of ceftazidime pivoxil hydrochloride. Add 200ml of saturated sodium chloride solution at a temperature of -15°C dropwise to the memethicone ethanol solution at a constant speed, and the dropwise addition is completed within 0.5 hours at a constant speed. Leave for 4 hours to precipitate pale yellow crystals, filter, wash with distilled water and ethyl acetate successively, and then vacuum-dry to obtain 9.78 g of ceftazidime hydrochloride hydrate crystals.
[0040]Det...
Embodiment 2
[0048] Dissolve 10 g of the crude ceftazidime hydrochloride crude drug in 50 ml of ethanol at a temperature of 10°C, add activated carbon with a crude quality of 0.02 for ceftazidime hydrochloride to decolorize for 30 minutes, filter, and wash with a small amount of ethanol solution to obtain ceftazidime hydrochloride Ethanol solution, the ethanol solution of ceftazidime hydrochloride was heated to 30 °C, and 350 ml of saturated sodium chloride solution with a temperature of -10 °C was added dropwise to the ethanolic solution of ceftazidime hydrochloride at a stirring rate of 23 rmp for 0.5 h. The dropwise addition was completed at a constant speed. After the dropwise addition was completed, the temperature was lowered to -10 °C and the stirring was continued for 1 h at a stirring speed of 12 rmp. After standing for 2 h, light yellow crystals were precipitated. 9.89 g of crystals of US ester hydrate.
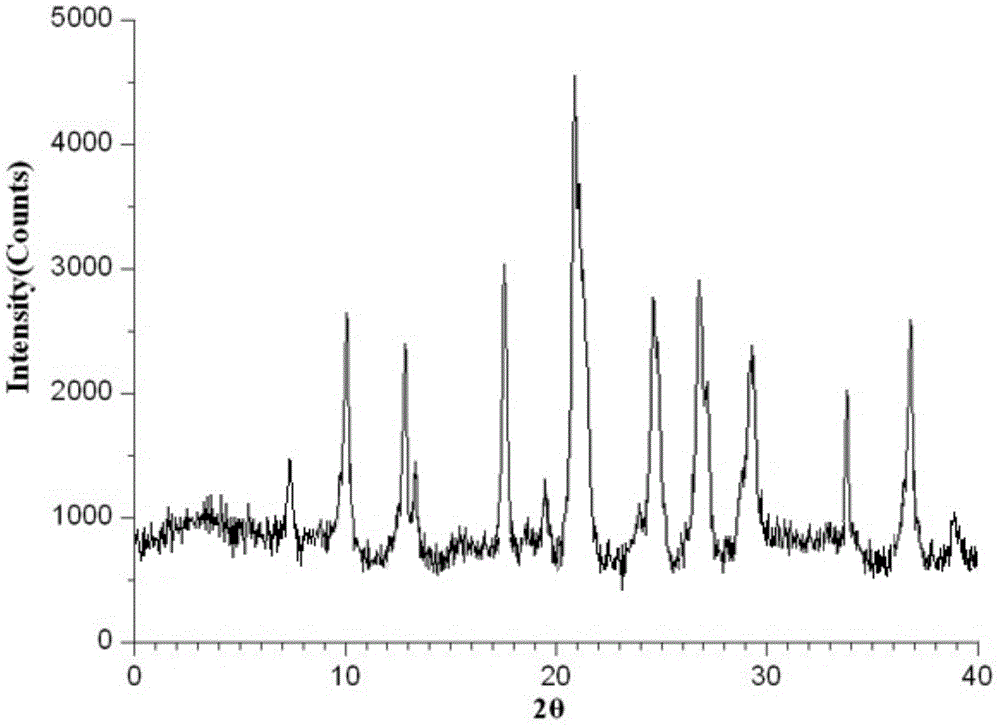
[0049] Determined by powder X-ray diffractometry, the X-ray powder diffract...
Embodiment 3
[0053] Dissolve 10 g of the crude ceftazidime hydrochloride crude drug in 80 ml of ethanol at a temperature of 8 °C, add activated carbon with a crude quality of 0.1 ceftazidime hydrochloride to decolorize for 25 minutes, filter, and wash with a small amount of ethanol solution to obtain ceftazidime hydrochloride Ethanol solution, the ethanol solution of ceftazidime hydrochloride was heated to 25 °C, and 500 ml of saturated sodium chloride solution at a temperature of -5 °C was added dropwise to the ethanolic solution of ceftazidime hydrochloride at a stirring rate of 25 rmp for 0.5 h. The dropwise addition was completed at a constant speed. After the dropwise addition was completed, the temperature was lowered to -10°C and the stirring was continued for 2 hours at a stirring rate of 15 rpm. After standing for 4 hours, light yellow crystals were precipitated. 9.83 g of US ester hydrate crystals.
[0054] Determined by powder X-ray diffractometry, the X-ray powder diffraction p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com