Synthesis method of polymerization catalyst of polyamine epoxy compounds
A technology for epoxy compounds and polymerization catalysts, applied in the direction of phosphorus organic compounds, etc., can solve the problems of difficult removal, poor stability, and difficult purification, and achieve the effects of simplified post-processing, good stability, and low unsaturation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The invention relates to a synthesis method of a polymerization catalyst of phosphine amine epoxy compounds, belonging to the technical field of compound synthesis. The present invention relates to a synthesis method of a more efficient phosphine amine polymerization catalyst, including the use of different raw materials, solvents, temperatures, and time to improve the reaction yield, and the intermediates are easier to purify, and the multi-step reaction is completed in one step. The method Including the following steps:
[0020] Step 1, preparation of imidophosphorus. Make 1mol of phosphorus pentachloride (PCl 5 ) and 6~10mol of secondary amines (HNR 2 ) reaction, and then with 1~5mol of ammonia (NH 3 ) reaction, neutralize the resulting product with alkali to obtain the crude product of imido-orthophosphorus, which can be obtained through vacuum distillation with high purity imido-orthophosphorus. Its structural formula is as follows (2)
[0021]
[0022] (2) ...
Embodiment 1
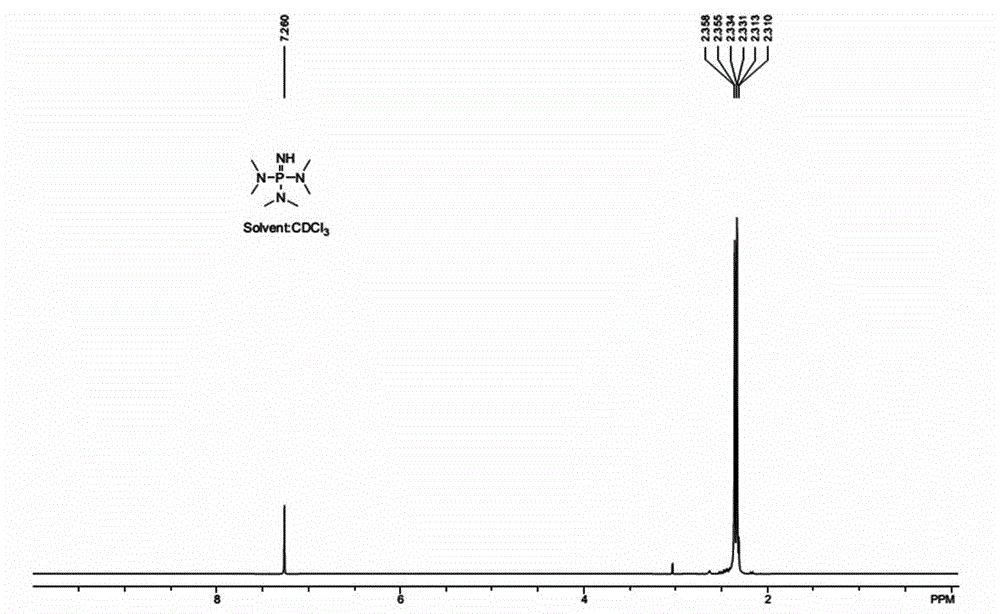
[0047]Phosphorus pentachloride (208 g, 1.0 mol) was added to 300 mL of dichloromethane under the protection of nitrogen, cooled to -30 °C in a dry ice bath, and slowly added dropwise into dichloromethane (600 mL of dimethylamine (300 g, 6.6 mol) ) solution, raised to 20°C and stirred for 1 hour after addition. Cool in a dry ice bath to -20°C, slowly introduce at least 85g of ammonia gas (5mol), slowly raise the temperature to 20°C, and stir for 6 hours. The generated ammonium chloride was removed by filtration, and the solvent was distilled off under reduced pressure. Suspend the crude product in 500ml of toluene, add 30ml of 30% aqueous sodium hydroxide solution, stir at 50°C for 3 hours, filter to remove insoluble matter, and concentrate under reduced pressure to remove the solvent. The crude product was distilled to obtain 115 g of iminotris(dimethylamino)phosphine, yield: 65%. 1 H-NMR such as figure 1 shown.
Embodiment 2
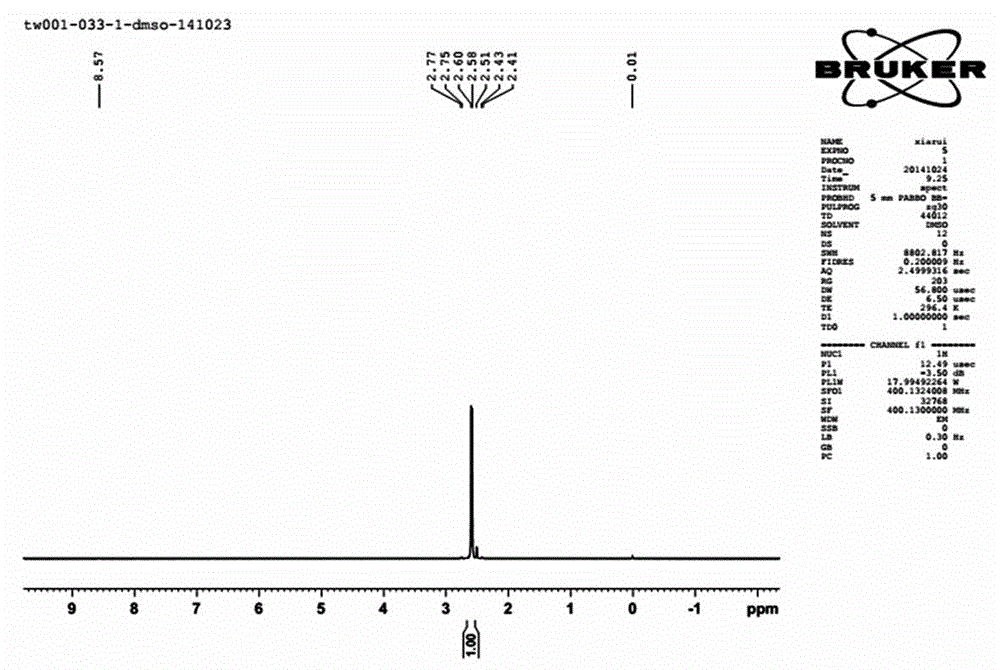
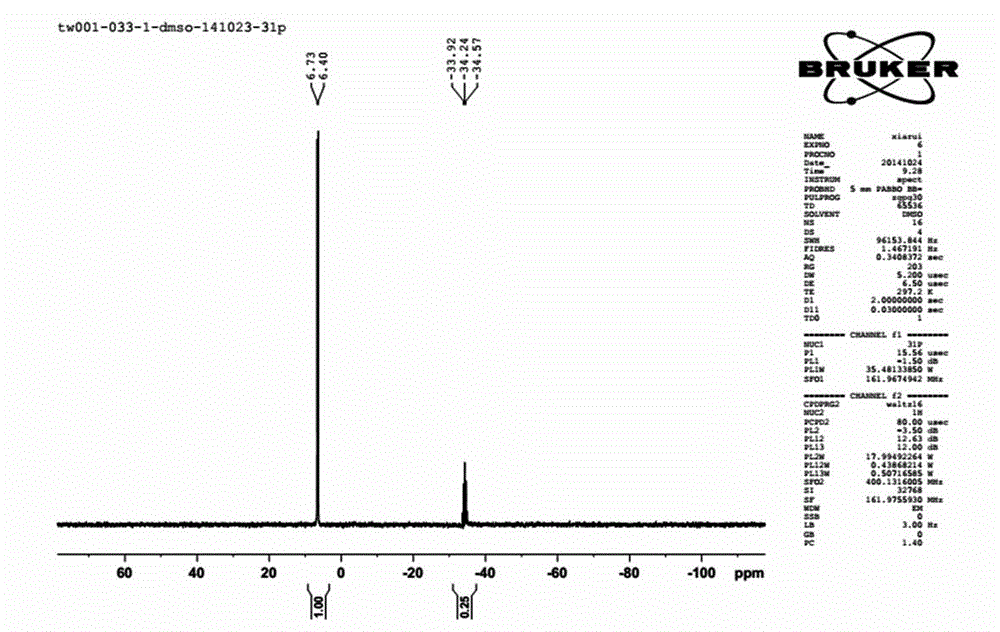
[0049] Phosphorus pentachloride (9.1g, 0.044mol) was suspended in 100 mL of xylene, the temperature was controlled at 40°C, and iminotris(dimethylamino)phosphine (66.7g, 0.375mol, after the dropwise addition, stir at 40°C for 1 hour, slowly raise the temperature to 120°C, and stir for 36 hours. Remove the generated iminotris(dimethylamino)phosphine hydrochloride by filtration, and remove the solvent by distillation under reduced pressure. 41 g of tetrakis[tris(dimethylamino)phosphoranylideneamino]phosphonium chloride were obtained. Yield: 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com