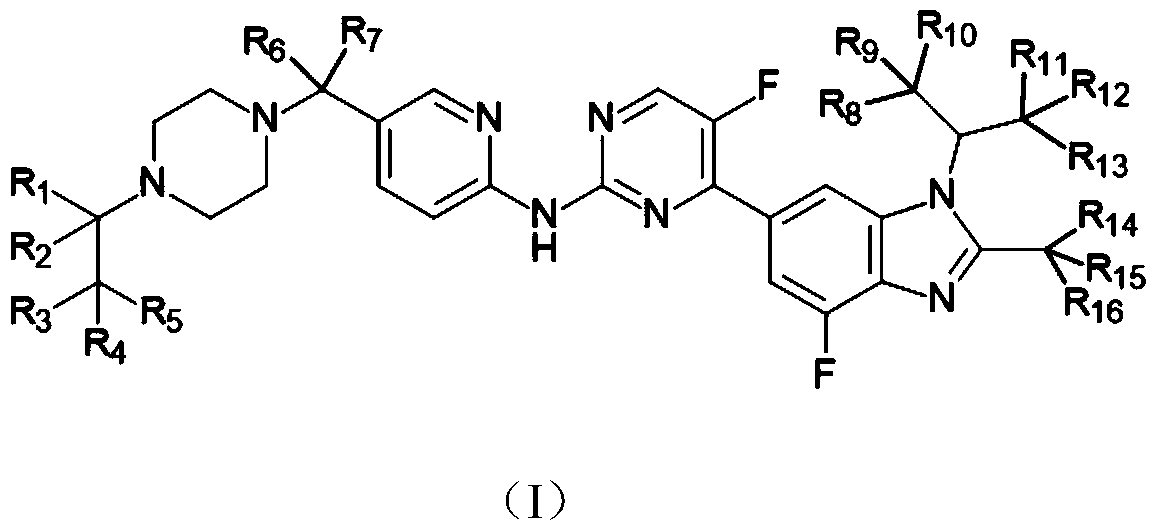
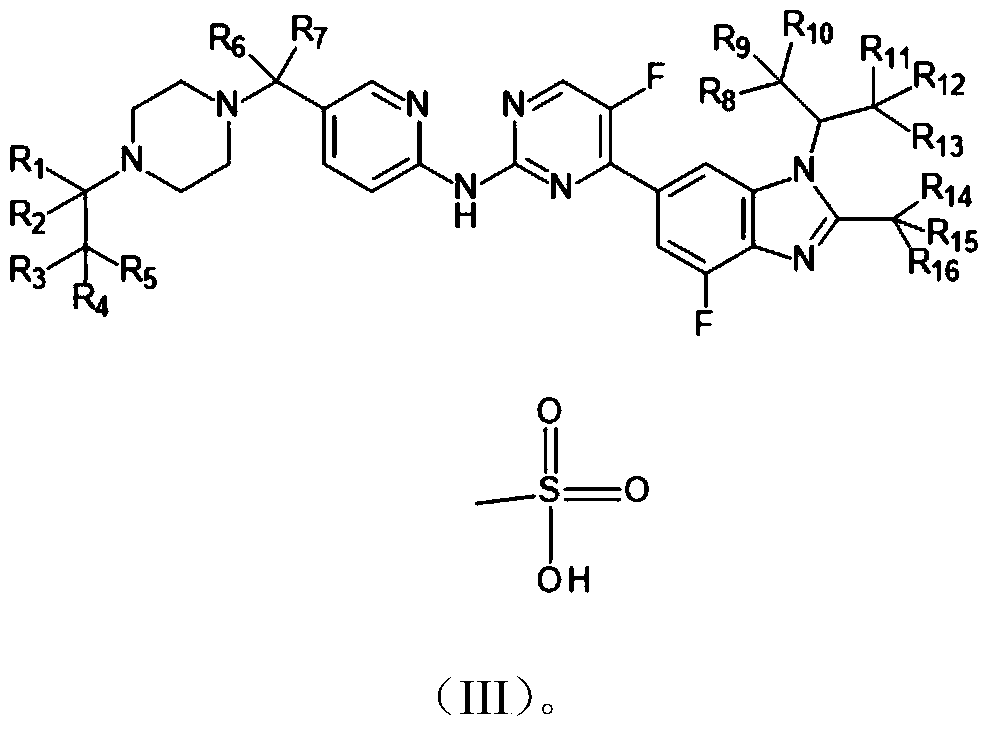
A kind of novel benzimidazole-pyrimidine amine derivative and its application
A technology of drugs and compounds, applied in the field of benzimidazole-pyrimidinamine derivatives, to achieve the effects of increasing concentration, reducing first-pass metabolism, reducing drug toxicity and other side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
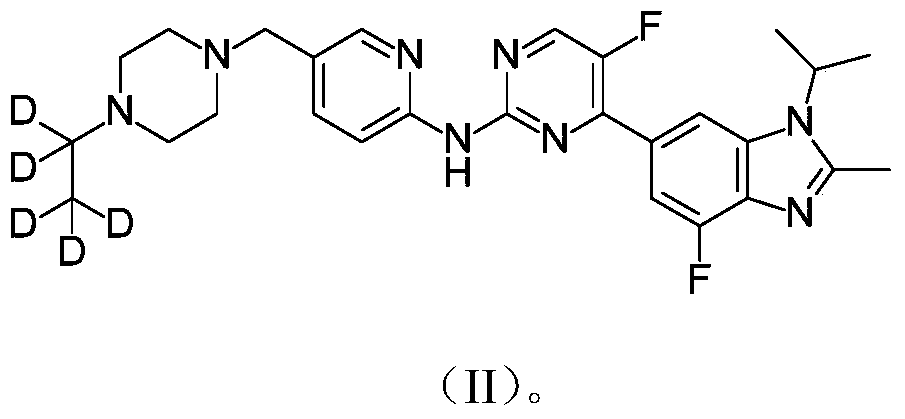
[0049] Preparation of [5-(4-pentadeuterioethyl-piperazin-1-ylmethyl)-pyridin-2-yl]-[5-fluoro-4-(7-fluoro-3-isopropyl-2- Methyl-3H-benzimidazol-5-yl)-pyrimidin-2-yl]-amine
[0050]
[0051] N 2 Under atmosphere, in 6-(2-chloro-5-fluoro-pyrimidin-4-yl)-4-fluoro-1-isopropyl-2-methyl-1H-benzimidazole (1.5g), 5-( 4-Pentadeuterioethyl-piperazin-1-ylmethyl)-pyridin-2-ylamine (1.1 g) in 1,4-dioxane (20 mL), was added cesium carbonate (3.0 g) in portions , tris(dibenzylideneacetone)dipalladium (0.2 g), and 4,5-bis(benzoylthio)-9,9-dimethylxanthene (0.2 g). The mixture was heated at 110° C. for 2 hours, and the reaction was monitored by TLC. After cooling to room temperature, the reaction solution was poured into ice water (50 mL) with stirring. This system was extracted with dichloromethane (30 mL X 3). The organic phases were combined, washed with brine (20 mL X 2), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 1.8 g of a brown viscous ...
Embodiment 1b
[0054] Preparation of [5-(4-pentadeuterioethyl-piperazin-1-ylmethyl)-pyridin-2-yl]-[5-fluoro-4-(7-fluoro-3-isopropyl-2- Methyl-3H-benzimidazol-5-yl)-pyrimidin-2-yl]-amine methanesulfonate
[0055]
[0056] N 2 Under atmosphere, in [5-(4-pentadeuteroethyl-piperazin-1-ylmethyl)-pyridin-2-yl]-[5-fluoro-4-(7-fluoro-3-isopropyl -2-Methyl-3H-benzimidazol-5-yl)-pyrimidin-2-yl]-amine (0.7 g) in dichloromethane (10 mL) and methanol (10 mL) was added methanesulfonic acid ( 10mL). The solution was stirred at room temperature for 1 hour and the solvent was removed. After washing several times with methyl tert-butyl ether, 0.9 g of the title compound was obtained.
[0057] 1 H-NMR (CD 3 OD-d4)δ8.58(b,1H),8.32(s,1H),8.26(b,2H),7.87-7,78(m,2H),4.98-4.92(m,1H),3.67(brs ,2H), 3.50-3.42(m,2H), 3.22-3.09(m,6H), 2.71(d,6H), 1.72(d,6H).
[0058] Preparation of intermediate 5-(4-pentadeuteroethyl-piperazin-1-ylmethyl)-pyridin-2-ylamine
[0059] N 2 Under atmosphere, in a degassed solut...
Embodiment 2a
[0068] Preparation of [5-(4-(2,2,2-trideuteroethyl)-piperazin-1-ylmethyl)-pyridin-2-yl]-[5-fluoro-4-(7-fluoro- 3-isopropyl-2-methyl-3H-benzimidazol-5-yl)-pyrimidin-2-yl]-amine
[0069]
[0070] It was prepared according to the method described in Example 1, except that 1-(2,2,2-trideuteroethyl)piperazine was used instead of 1-pentadeuteroethylpiperazine to obtain the target product.
[0071] MS (ES+): m / z = 510.2 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com