Bilobalide K derivative and preparation method and application thereof
A technology of drugs and compounds, applied in the field of new ginkgolide K derivatives, can solve the problems of poor bioavailability, insoluble in water, and limitation of drug efficacy, and achieve simple preparation process, high activity, and water solubility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
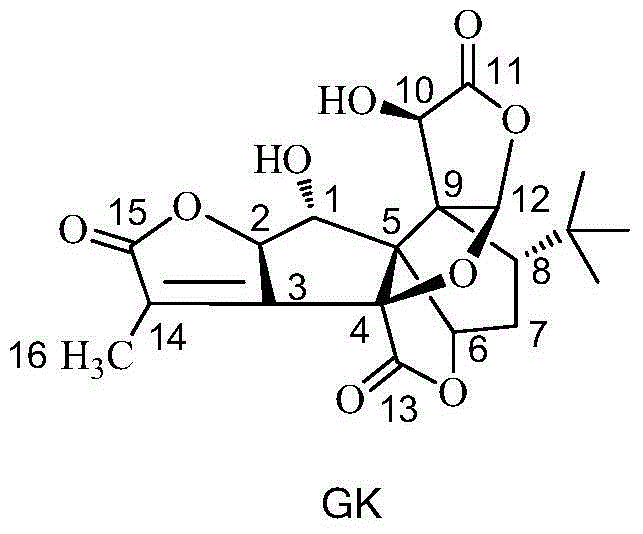
[0056] The preparation of embodiment 1 ginkgolide K
[0057] Dissolve 50 g of ginkgolide B in 100 mL of dry dichloromethane, stir and mix evenly, then cool down to -25°C under nitrogen protection. Add 200mL DAST dropwise (that is, the volume-to-weight ratio of ginkgolide B is 4mL / g), and keep it warm for 0.5h; Add 500mL of purified water to the reaction solution, quench the reaction and remove the organic solution by rotary evaporation, extract the water layer with ethyl acetate, wash the organic layer with saturated sodium bicarbonate solution and sodium chloride solution, and then dry it, then remove the organic solution by rotary evaporation to obtain Oil. Column chromatography purification (V 二氯甲烷 / V 甲醇 =20:1) to obtain white solid ginkgolide K altogether 12.5g, yield 26.15%, HPLC purity is 99.20%;
[0058] LC-MS: 407.2 [M+H + ], 835.3[2M+Na + ].
[0059] 1 H-NMR (DMSO, 400MHz): 1.04 (s, 9H, t-Bu), 2.16-2.20 (dd, 1H, 8-H), 1.86-1.88 (m, 5H, -CH3; 7-H), 3.82 -3.85(...
Embodiment 2
[0060] Example 2 Preparation of 10-O-(dimethylaminoethyl) ginkgolide K (GK-1)
[0061] 2.0g ginkgolide K was dissolved in 50mL acetonitrile, and 0.85g dimethylamino ethyl chloride hydrochloride (1.2eq), 6.81g potassium carbonate (10eq) and 0.82g KI catalyst (1.0eq) were added successively, in React at 30°C until the reaction of the raw material ginkgolide K is complete. Cool to room temperature, filter and remove the filtrate by rotary evaporation to obtain a pale yellow oil. Column chromatography purification (V 石油醚 :V 乙酸乙酯 =2:1) After that, 0.67g of GK-1 was obtained as a white solid, with a yield of 28.51% and a purity of 99.40% by HPLC.
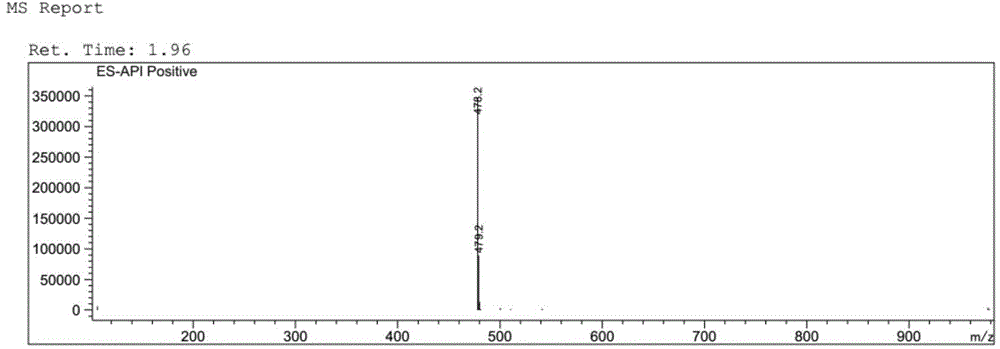
[0062] LC-MS: 478.2 [M+H + ].
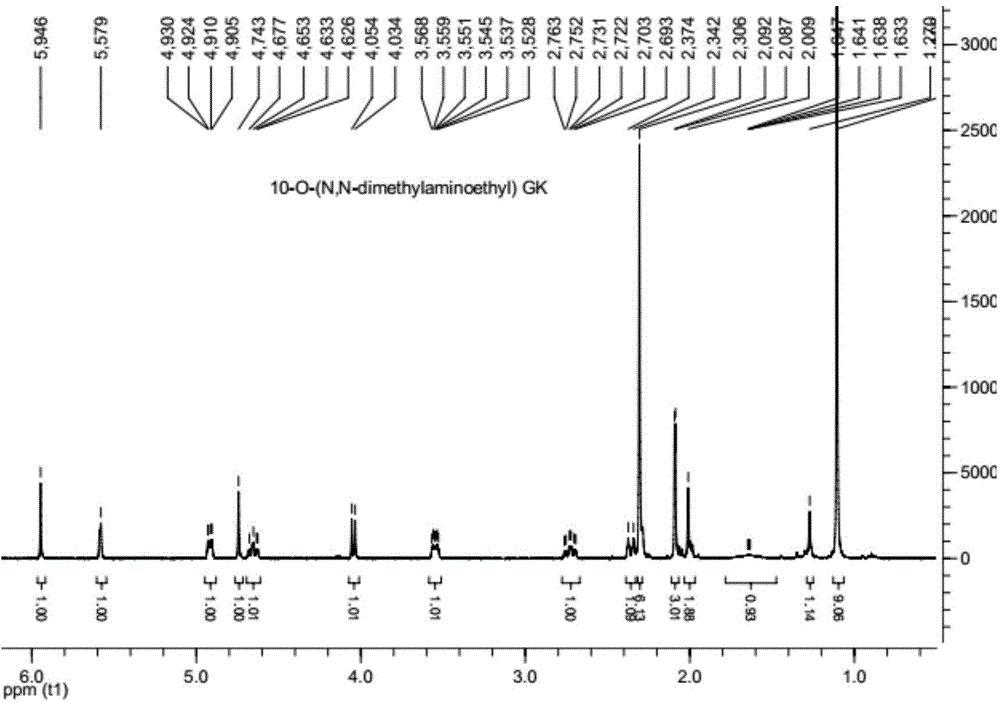
[0063] 1 H-NMR (CDCl 3 , 400MHz): 1.11(s,9H,t-Bu),1.63-1.64(dd,1H,8-H),2.08-2.09(s,3H,14-CH 3 ),2.00(s,2H,7-H),2.30(s,6H,(CH 3 ) 2 N-),1.27,2.34-2.37,2.69-2.76,3.52-3.56(s,d,mx2,1Hx4,NCH 2 CH 2 O), 4.03-4.05(d, 1H, 1-H), 4.62-4.65(m, 1H, 1-OH), 4.67(s, 1H, 10-H), 4.74(dd, 1H, 6-H) , 5.57 (s, ...
Embodiment 3
[0064] Example 3 Preparation of 10-O-(aminoethyl) ginkgolide K (GK-2)
[0065] Dissolve 2.0g ginkgolide K in 50mL acetonitrile, add 0.68g 2-chloroethylamine hydrochloride (1.2eq), 6.81g sodium carbonate (10eq) and 0.82g KI catalyst in sequence, and react at 60°C until The reaction of raw material ginkgolide K is completed. Cool to room temperature, filter and remove the filtrate by rotary evaporation to obtain a light yellow solid. Column chromatography purification (V 石油醚 :V 乙酸乙酯 =1:1) to obtain 0.50 g of GK-2 as a white solid with a yield of 22.62%. HPLC purity 99.13%.
[0066] LC-MS: 450.20 [M+H + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com