Preparation method of glufosinate and analogue of glufosinate
An analog, glufosinate-ammonium technology, which is applied in the field of preparation of glufosinate-ammonium and its analogs, can solve the problems of difficult glufosinate-ammonium separation and purification process, cumbersome purification process, a large amount of ammonium chloride, etc., and achieves high product purity and yield. High, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
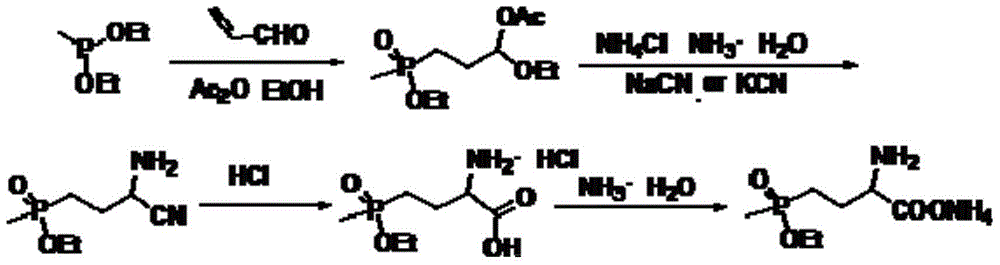
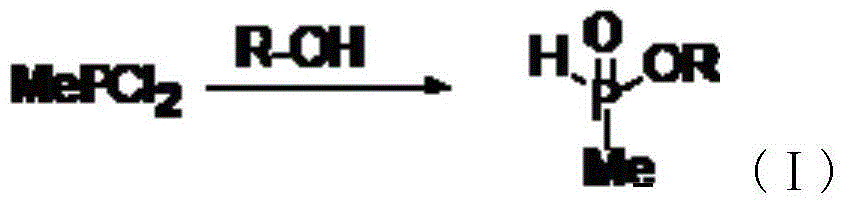
[0026] In a 500ml four-neck flask, add 5.8gKOH, 95g methyl phosphine dichloride, 180ml n-butyl ester, and the reaction temperature is 210°C. Small molecules are continuously separated during the reaction, and 200ml N-N-dimethylformamide is added after the reaction is completed. Liquid separation was carried out in a separatory funnel to obtain 108 g of intermediate (I), with a purity of 99.2%.
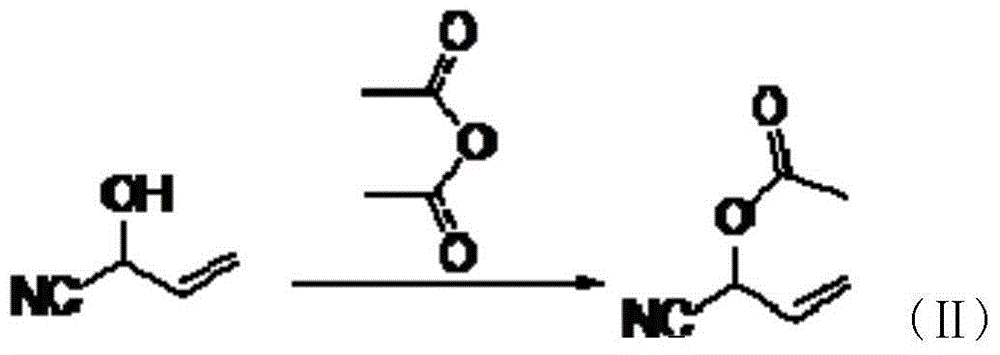
[0027] Add 220ml of formic acid to the intermediate (I), add 6.7g of catalyst KOH, put in 66.2g of 2-hydroxybutene-3-carbonitrile, slowly add 135ml of acetic anhydride into the constant pressure dropping funnel, and control the dropping time between 30min and 40min After the reaction was completed, the small molecules were distilled off under reduced pressure to obtain 88.6 g of intermediate (II) with a purity of 98.5%.
[0028] In the intermediate (II), add 66 g of n-butyl methylphosphonite, 88.6 g of 2-acetoxy-3-butenenitrile, add 0.20 g of benzoyl peroxide, and react for 2 hours at ...
Embodiment example 2
[0031] In a 500ml four-neck flask, add 4.2gKOH, 65g methyl phosphine dichloride, 120ml n-butyl ester, and the reaction temperature is 230°C. Small molecules are continuously separated during the reaction, and 150ml N-N-dimethylformamide is added after the reaction is completed. Liquid separation was carried out in a separatory funnel to obtain 78 g of intermediate (I) with a purity of 99.5%.
[0032] To the intermediate (I), add 180ml of formic acid, add 4.2g of catalyst KOH, put in 56.8g of 2-hydroxybutene-3-carbonitrile, slowly add 95ml of acetic anhydride into the constant pressure dropping funnel, and the dropping time is controlled within 30min~ After 40 minutes, the small molecules were distilled off under reduced pressure to obtain 66.6 g of intermediate (II) with a purity of 99.0%.
[0033] In the intermediate (II), add 66.6 g of n-butyl methylphosphonite, drop 78 g of 2-acetoxy-3-butenenitrile, add 0.20 g of benzoyl peroxide, and react for 2 hours at 70° C. After com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com