In-vitro screening method for antineoplastic activity of artemisinin and derivatives of artemisinin
A technology for anti-tumor activity and in vitro screening, applied in the field of in vitro screening of anti-tumor activity of artemisinin and its derivatives, can solve the problems of high cost and long time, and achieve the effects of low cost, short time and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
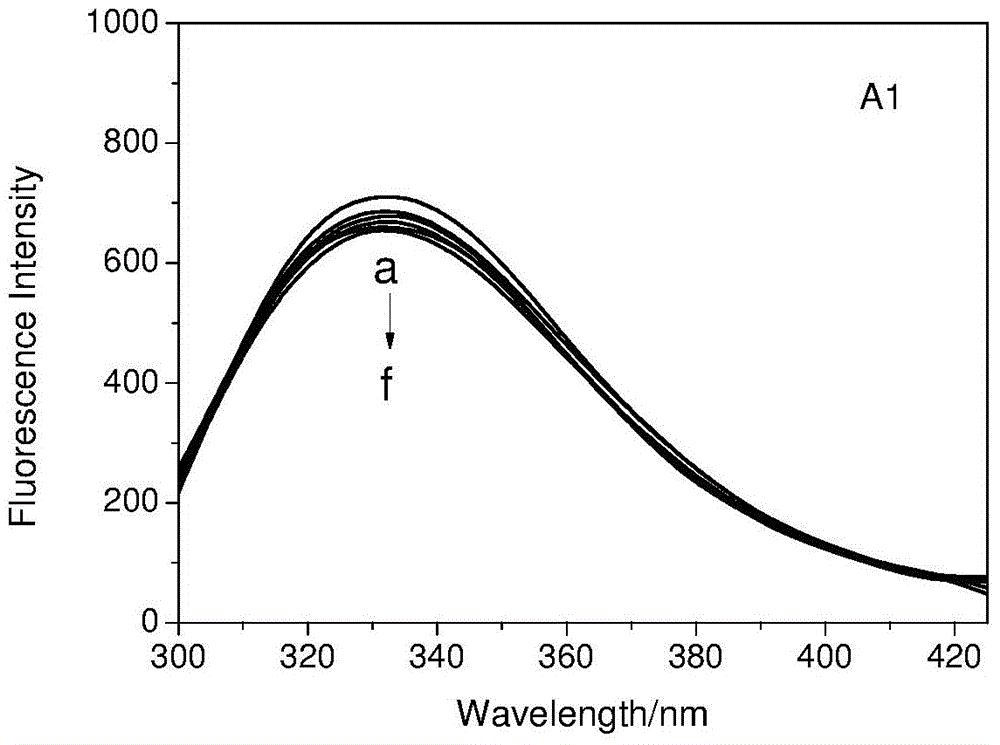
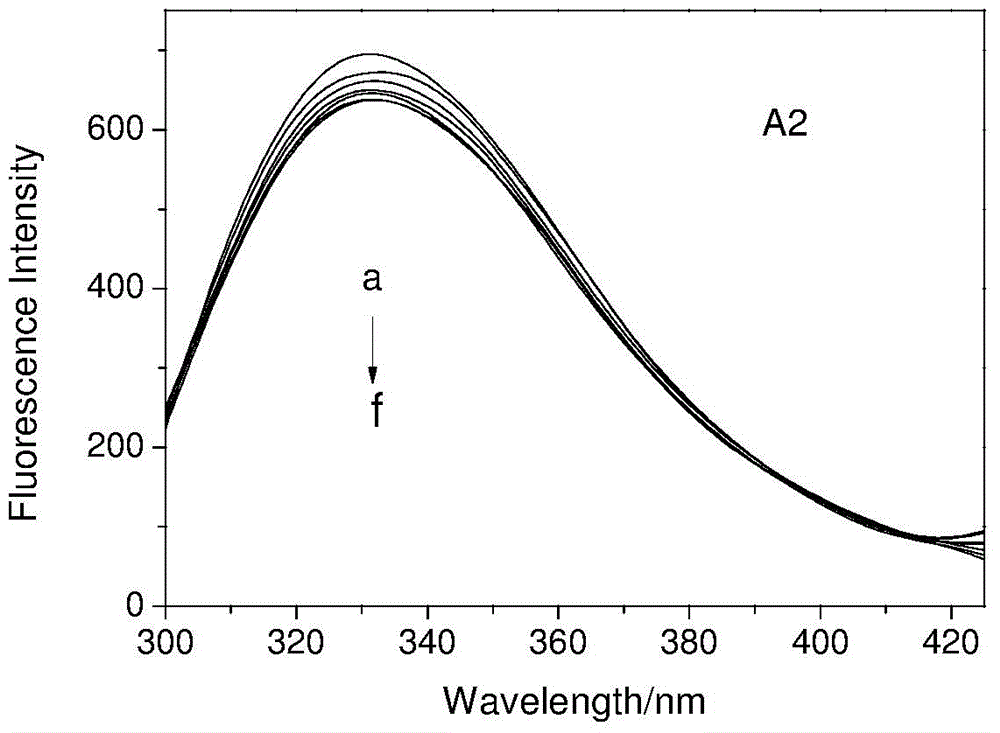
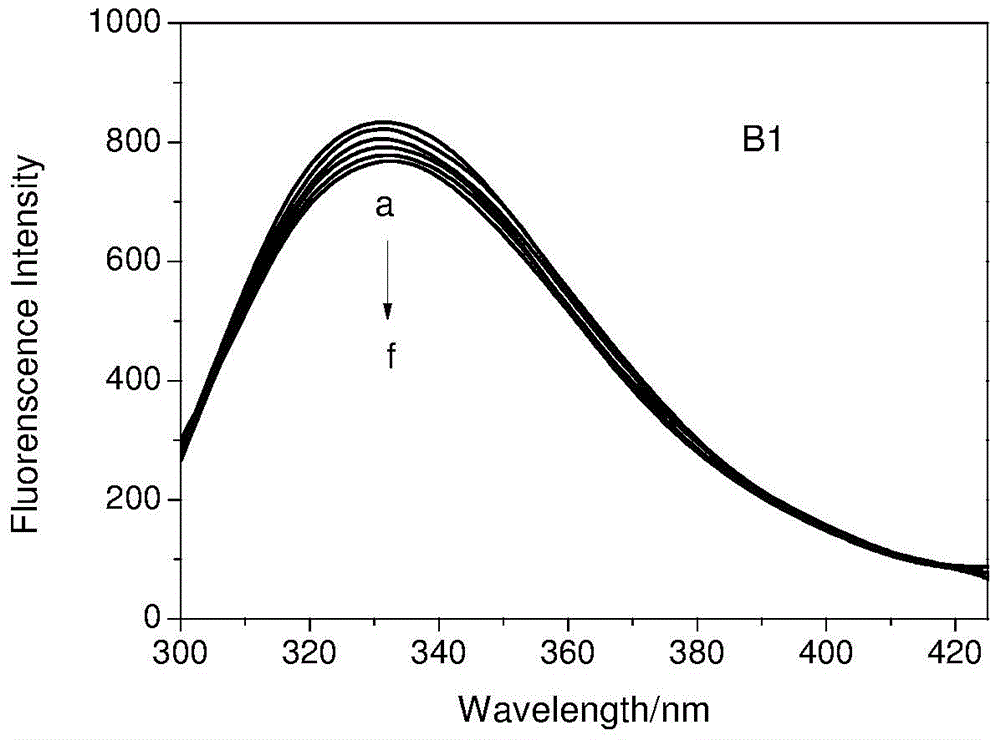
[0028] In the concentration range of 1.0×10 -6 ~1×10 -4 Configure transferrin solution in mol / L, fix the concentration of transferrin, add artemisinin, dihydroartemisinin and 9-OH artemisinin respectively, in the concentration range of 1.0×10 -6 ~1×10 -3 Gradually increase the concentration of artemisinin, dihydroartemisinin (DHA) and 9-OH artemisinin (9-OH QHS) in the solution within mol / L, and scan at 298K and 310K with an excitation wavelength of 280nm. The fluorescence emission spectrum of the system is shown in Figure 1.
[0029] The binding constant K can be calculated from the following equation b And the number of binding sites n
[0030] log[(F 0 -F) / F]=log K b +n log[Q]
[0031] Where: F 0 And F respectively represent the fluorescence intensity of transferrin in the absence and presence of the quencher; [Q] is the concentration of artemisinin derivatives.
[0032] Linear regression method can be used, artemisinin derivatives quench the fluorescence of transferrin lg[(F 0 -F)...
Embodiment 2
[0039] Example 2 is basically the same as Example 1, but the artemisinin derivative is artemether or ether.
Embodiment 3
[0040] Example 3 is basically the same as Example 1, but the artemisinin derivatives are artesunate, artemisinin carbonate and artemisinin carboxylate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com