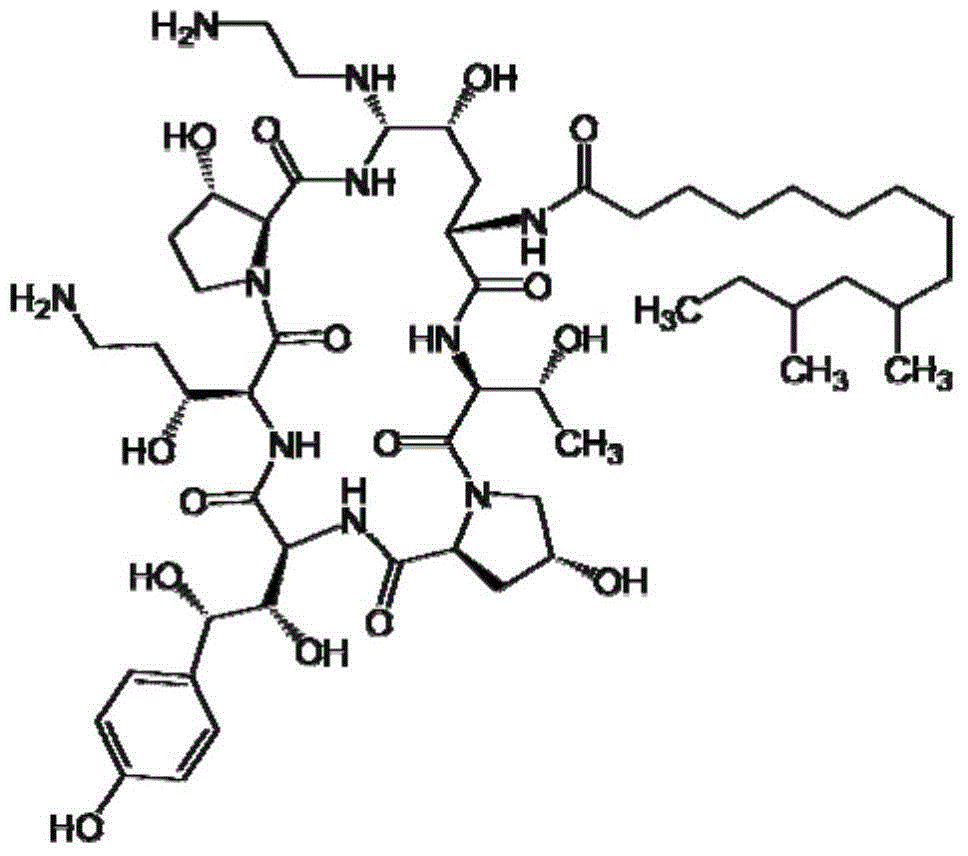
Method for finely purifying caspofungin
A caspofungin, fine technology, applied in the preparation method of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problem that the purity of caspofungin does not meet the high The quality cannot be well separated and controlled, and the process is complicated, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] by Finely purify caspofungin as a chromatographic medium: use a 15×310mm glass chromatographic column, (produced by Suzhou Nawei Technology Co., Ltd.) is a chromatographic column packed with chromatographic packing material, and the column volume is 55ml. 0.5 g of crude caspofungin (purity 91%) was dissolved in 8 ml of water, and filtered through a 0.45 μm filter membrane for later use. The above-mentioned caspofungin solution is loaded into the chromatographic column, washed with 21% aqueous acetonitrile (containing 0.03%-0.2% acetic acid) at 7ml / min for 12 column volumes (BV), and the eluted Caspofungin, through HPLC analysis, the peak area of caspofungin in the eluent is 99.8% of the total area, the single-component impurity is less than 0.1%, and the recovery rate is 61.8%.
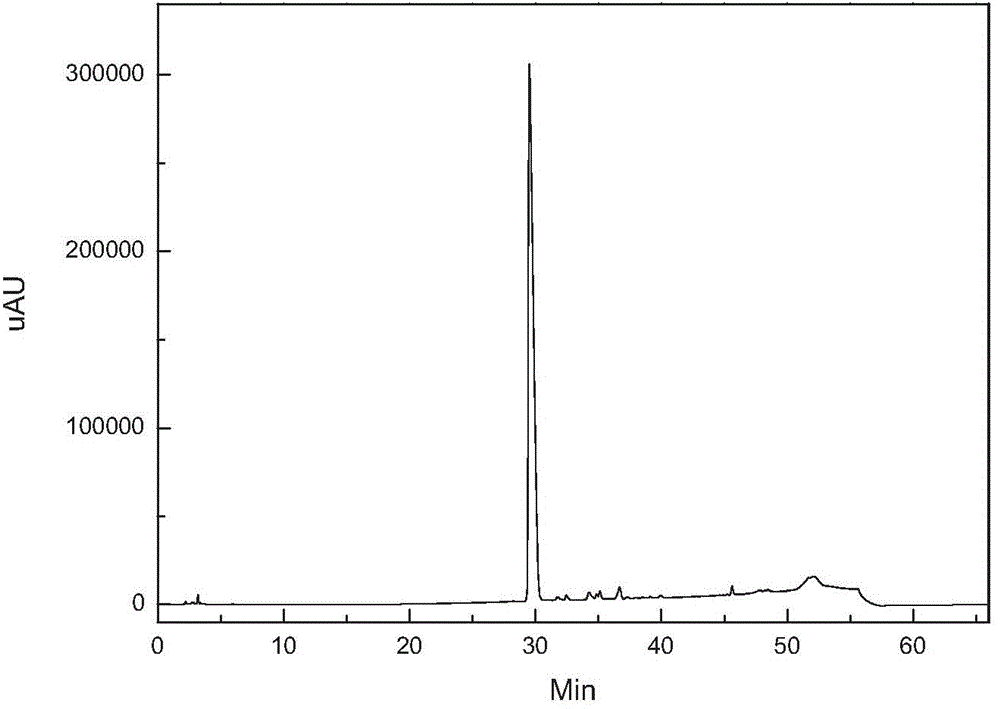
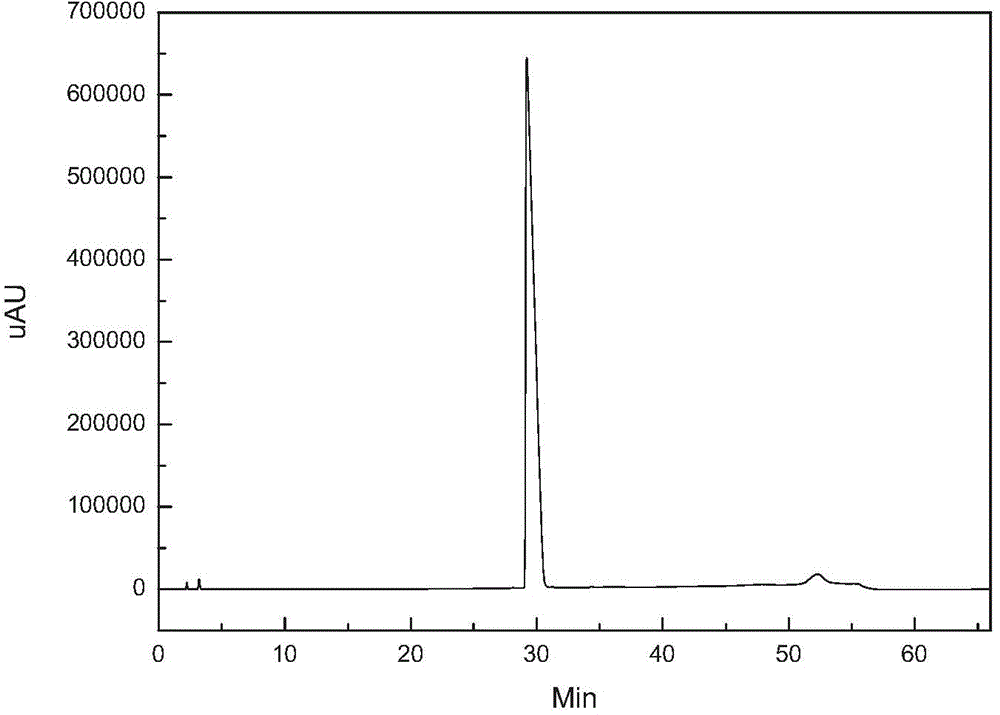
[0021] figure 1 It is the HPLC analysis spectrum of caspofungin crude product before purification. It can be seen that there are many impurities and the content is high. figure 2 It i...
Embodiment 2
[0023] by Finely purify caspofungin as a chromatographic medium: use a 15×310mm glass chromatographic column, (C18 reversed-phase silica gel filler, produced by Suzhou Nawei Technology Co., Ltd.) was used to fill the chromatography column with a packing volume of 55 ml. 0.5 g of crude caspofungin (purity 91%) was dissolved in 8 ml of water, and filtered through a 0.45 μm filter membrane for later use. Say above-mentioned caspofungin solution loads in the chromatographic column, wash 16 column volumes (BV) with 21% acetonitrile aqueous solution with 7ml / min, collect the caspofungin that is eluted, analyze through high performance liquid phase , the peak area of caspofungin in the eluate is 97.6% of the total area, the single-component impurity is less than 0.15%, and the recovery rate is 60.2%.
Embodiment 3
[0025] by Finely purify caspofungin as a chromatographic medium: use a 15×310mm glass chromatographic column, (C18 reversed-phase silica gel filler, produced by Suzhou Nawei Technology Co., Ltd.) was used to fill the chromatography column with a packing volume of 55 ml. 0.5 g of crude caspofungin (purity 91%) was dissolved in 8 ml of water, and filtered through a 0.45 μm filter membrane for later use. Say above-mentioned caspofungin solution loads in the chromatographic column, wash 16 column volumes (BV) with 21% acetonitrile aqueous solution with 7ml / min, collect the caspofungin that is eluted, analyze through high performance liquid phase , the peak area of caspofungin in the eluate is 96.5% of the total area, the single-component impurity is less than 0.15%, and the recovery rate is 60.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com