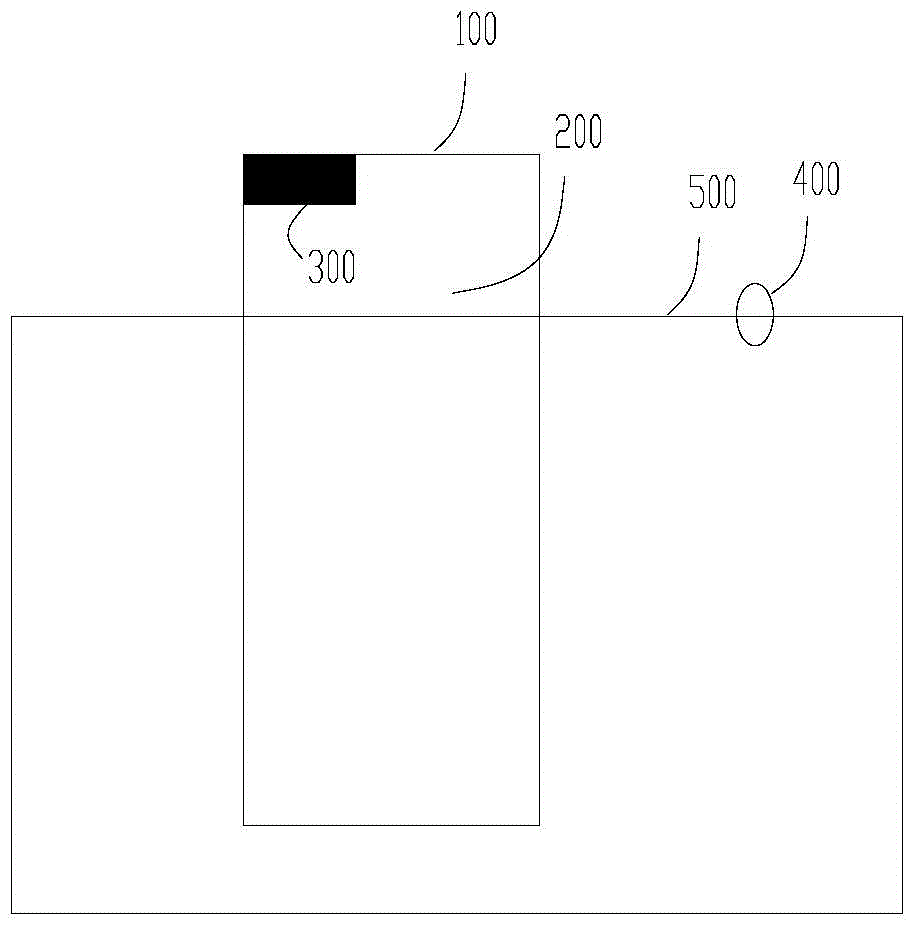Systems for Preparing Compounds
A compound and equipment technology, applied in the chemical/physical/physicochemical process of energy application, can solve problems that need to be improved, and achieve the effect of easy operation, easy operation, and good light quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] In this example, hypericin was synthesized by the following steps using the system used to prepare the compound:
[0067] (1) Add 840 mg (3 mmol) of emodin and 50 mL of glacial acetic acid in a four-necked bottle, N 2 protection, add SnCl under stirring 2 2H 2 O 2.84g (12.6mmol), after warming up to reflux, slowly add 5mL of 48% hydrobromic acid dropwise (in 4 drops at intervals of 30min); after addition, continue to react for 2h (TLC tracking). Cool to room temperature, filter with suction, wash the filter cake with water until neutral, and dry to obtain 2672 mg of light yellow powder;
[0068] (2) Add the powder 2512mg (2mmol) that step (1) obtains in four-necked bottle successively, pyridine 10mL, FeSO 4 ·7H 2 O 820mg (0.295mmol), pyridine nitrogen oxide 1.25g (13.2mmol) and pyridine 1 mL, N 2 Protected from light and heated to reflux (100° C.) for 1.5 h (TLC tracking). Cool to room temperature, evaporate the organic solvent, add 200mL of 3% hydrochloric acid, ...
Embodiment 2
[0073] In this example, 2-bromo-2-(2-fluorophenyl)-1-cyclopropylethanone was synthesized by the following steps using the system used to prepare the compound:
[0074] In a 250mL three-necked round bottom flask equipped with a stirrer and a thermometer, add 10.0g (56.1mmol) of 2-(2-fluorophenyl)-1-cyclopropylethanone, 100mL of dichloromethane, dibromohydantoin (8.0 g, 28.0 mmol), benzoyl peroxide (0.5g), at room temperature, react 4h under the illumination of 15W sight light, then cool, concentrate and reclaim methanol, the residue is dissolved in dichloromethane, filters, and the filtrate Washed to neutral, anhydrous MgSO 4 Dry and concentrate under reduced pressure to constant weight to obtain light yellow oil.
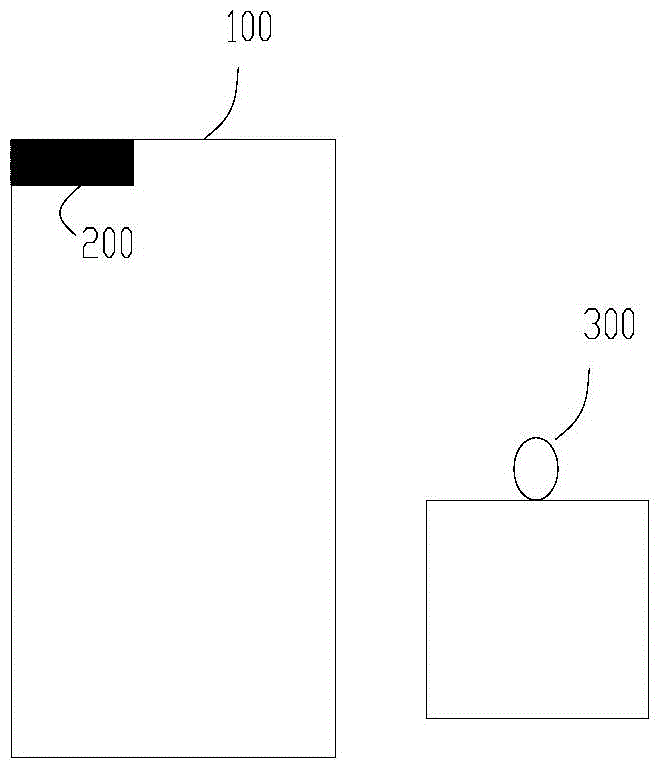
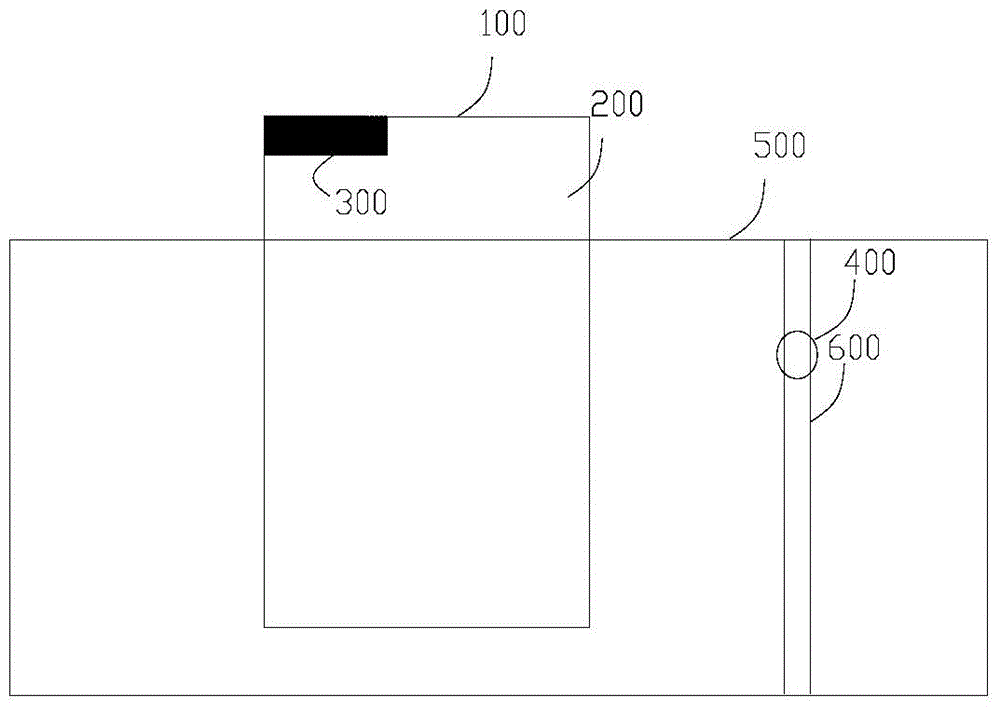
[0075] The height of the liquid level limiter of the photoreaction equipment from the bottom of the reaction vessel is H, and the average distance between the light source and the vertical axis of the reaction vessel is R2, wherein H and R2 satisfy the relational f...
Embodiment 3
[0078] In this example, a novel oleanolic acid derivative was synthesized by the following steps using the system used to prepare the compound:
[0079] (1) Add 60mL of diethyl ether and 20ml of 40% KOH solution to the flask, add 6g of methylnitrosourea in batches under stirring at below 5°C; react for 10min, separate the ether layer to obtain CH 2 N 2 Diethyl ether solution, dried with KOH for later use. Add 110g (21.9mmol) of THF (20mL) solution into the reaction flask, add CH 2 N 2 Diethyl ether solution, after dropping, reacted to the end point (monitored by TLC). The solvent was evaporated, the residue was dried and CHCl was added 3 50ml and pyridine 30mL, add Ac 2 O 10mL, after addition, react at room temperature to the end. Water, 5% HCl solution, saturated NaHCO 3 Solution, water and saturated brine washing, anhydrous MgSO 4 dry. After distilling off the solvent, recrystallize with ethyl acetate to obtain white crystals;
[0080] (2) Add dry CH successively t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com