Preparing method for improving LiH-NH<3> hydrogen storing system dehydrogenizing kinetics
A kinetic and system technology, applied in chemical instruments and methods, hydrogen, inorganic chemistry, etc., can solve problems such as slow reaction kinetics and hinder practical application, and achieve the effect of accelerating hydrogen evolution reaction and improving dehydrogenation kinetics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
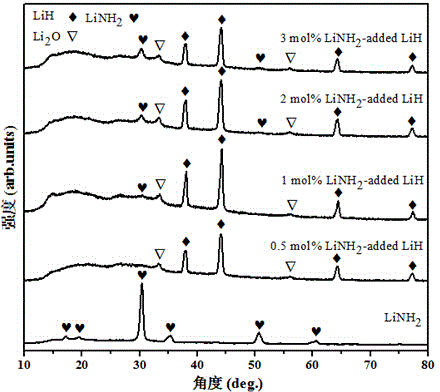
[0021] 1. Ball mill LiH sample: In a vacuum glove box, weigh 0.3g LiH crystal sample and put it into a ball mill jar containing 30 stainless steel balls. Then the ball mill jar was taken out from the glove box, filled with a certain amount of hydrogen, and the ball mill jar was symmetrically installed in a planetary ball mill, and ball milled at a speed of 350 rpm for 2 hours.
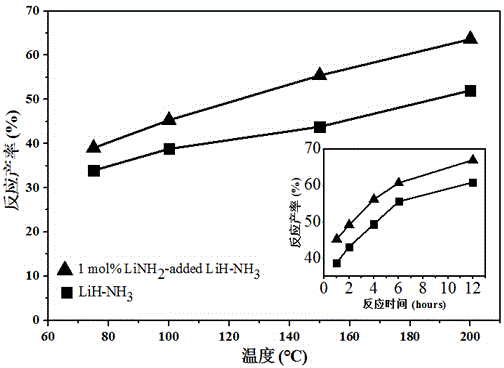
[0022] 2. Sampling and ammonia gas for hydrogen desorption reaction: In a vacuum glove box, accurately weigh 0.0191gLiH crystal sample in a stainless steel heating tube, install the stainless steel heating tube in the reaction device, and remove the gas circuit by vacuuming the gas circuit After removing the residual air, set the temperature controller parameters of the reaction to 100°C.
[0023] After the heating reactor reaches the set temperature, quickly fill it with 0.5MPa ammonia gas, react for 1 hour, stop the experiment, and immediately vent the gas in the pipeline. After the stainless steel h...
Embodiment 2
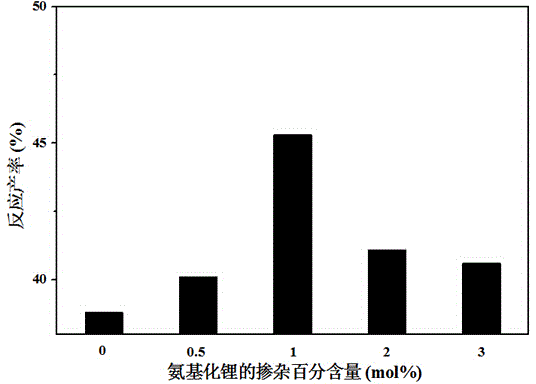
[0027] 1. Doped with 0.5mol% LiNH by ball milling 2 LiH sample: first for doping LiNH 2 The samples were pretreated by ball milling at 450 rpm for 24 hours. Then in the vacuum glove box, weigh 0.2957g LiH and 0.0043g LiNH 2 The samples were loaded into a ball mill jar containing 30 stainless steel balls. Then the ball mill jar was taken out from the glove box, filled with a certain amount of hydrogen, and the ball mill jar was symmetrically installed in a planetary ball mill, and ball milled at a speed of 350 rpm for 2 hours.
[0028] 2. Sampling and ammonia gas for dehydrogenation reaction: In a vacuum glove box, accurately weigh 0.0194g of the above-mentioned doped with 0.5mol% LiNH 2 LiH was placed in a stainless steel heating tube, and the stainless steel heating tube was installed in the reaction device. After vacuuming the gas circuit to remove the residual air in the gas circuit, set the parameters of the reaction temperature controller to 100 °C.
[0029] After h...
Embodiment 3
[0031] 1. Doped with 1 mol% LiNH by ball milling 2 LiH sample: In a vacuum glove box, weigh 0.2913 g LiH and 0.0087 g LiNH 2The samples (pretreated by ball milling) were loaded into a ball mill jar containing 30 stainless steel balls. Then the ball mill jar was taken out from the glove box, filled with a certain amount of hydrogen, and the ball mill jar was symmetrically installed in the QM-3SP4 planetary ball mill, and ball milled for 2 hours at a speed of 350 rpm.
[0032] 2. Sampling and ammonia gas for hydrogen desorption reaction: In a vacuum glove box, accurately weigh 0.0197 g of the above-mentioned doped sample in a stainless steel heating tube, and install the stainless steel heating tube in the reaction device, first clean the intake air of the reaction device After vacuumizing the gas path to remove the residual air in the gas path, set the parameters of the reaction temperature controller to 100 °C.
[0033] After heating to the set temperature of 100 °C, quickl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com