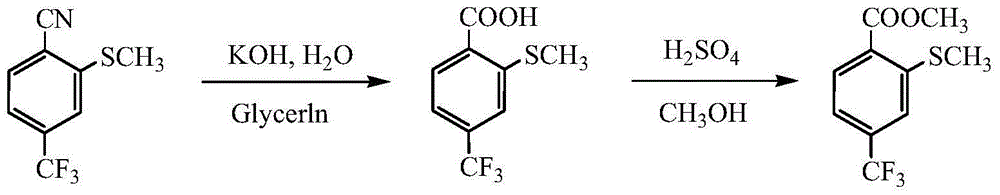
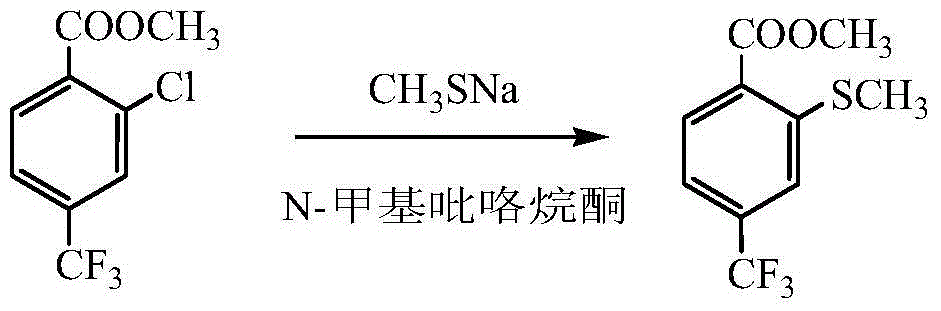
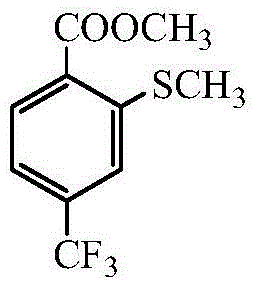Preparation method for 2-methylmercapto-4-thrifluoromethyl benzoate
A technology of methyl trifluoromethyl benzoate and trifluoromethyl benzonitrile, which is applied in the field of preparation of methyl 2-methylthio-4-trifluoromethyl benzoate, can solve the problem of high raw material cost and reaction conditions Harsh, unfriendly to the environment, etc., to achieve the effect of reducing the treatment of three wastes, shortening the reaction steps, and reducing the discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 100ml methanol in the reaction flask, then add 21.7g (0.1mol) 2-methylthio-4-trifluoromethylbenzonitrile, then add 22.6g90% aqueous sulfuric acid, heat the mixture at 80°C for 10 hours, and then The reaction solution was distilled to remove solvent methanol, the concentrated solution was washed with water, filtered, and dried to obtain 24.8g (0.0983mol) 2-methylthio-4-trifluoromethylbenzoic acid methyl ester, content 99.1%, yield 98.3% ( Calculated as 2-methylthio-4-trifluoromethylbenzocyanide).
Embodiment 2
[0026] Add 100ml of methanol in the reaction flask, then add 21.7g (0.1mol) 2-methylthio-4-trifluoromethylbenzonitrile, then add 22.6g of 60% sulfuric acid aqueous solution, after heating the mixture at 80°C for 10 hours, the The reaction solution evaporated methanol, and the concentrated solution was washed with water, filtered, and dried to obtain 24.7g (0.0967mol) 2-methylthio-4-trifluoromethylbenzoic acid methyl ester, content 97.9%, yield 96.7% (with 2- Methylthio-4-trifluoromethylbenzocyanide).
Embodiment 3
[0028] Add 100ml of methanol in the reaction flask, then add 21.7g (0.1mol) 2-methylthio-4-trifluoromethylbenzonitrile, then add 22.6g of 30% aqueous sulfuric acid, and heat the mixture at 80°C for 10 hours, The reaction solution was evaporated to methanol, the concentrated solution was washed with water, filtered and dried to obtain 24.0g (0.0933mol) 2-methylthio-4-trifluoromethylbenzoic acid methyl ester, content 97.2%, yield 93.3% (with 2 -methylthio-4-trifluoromethylbenzocyanide).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com