E.tenella TERT associated protein gene and medical uses thereof
A technology of Eimeria cocci and related proteins, applied in the field of genetic engineering, can solve problems such as proteins and functions that have not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] E. tenella Construction of TRBD bait expression plasmid
[0032] 1. Materials
[0033] Trizol (Invitrogen); reverse transcriptase kit (Tiangen); T4 DNA ligase, Ex Taq, dNTPs, pMD18-T, Eco R I, Bam H I (TaKaRa).
[0034] 2. Methods and results
[0035] 1 E. tenella Extraction of total RNA from sporulated oocysts
[0036] 1.1 Treatment of utensils: After washing the mortar and pestle, soak them in 0.1% DEPC-H 2 overnight in O. Then wrap it in tin foil and dry bake at 180°C for 3 hours for later use;
[0037] 1.2 Collected E. tenella Grind sporulated oocysts with liquid nitrogen for 20 minutes, add 1 mL Trizol, and lyse at room temperature for 5 minutes;
[0038] 1.3 Add 200 μL of chloroform, mix well, place at room temperature for 15 minutes, and centrifuge at 12000 g, 4°C for 15 minutes;
[0039] 1.4 Transfer about 600 μL RNA from the upper layer to a new centrifuge tube, add 500 μL isopropanol, mix well, place at room temperature for...
Embodiment 2
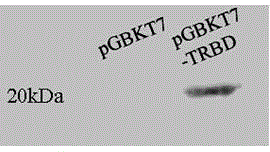
[0058] Detection of Toxicity and Self-activation of Recombinant Bait Protein on Yeast Strains
[0059] 1. Materials
[0060] X-α-GAL, salmon sperm DNA (Sigma); yeast nitrogen source (Biosharp); c-Myc (Epitomics); ECL chemiluminescence kit (Thermo); yeast plasmid mini-prep kit (Tiangen).
[0061] 2. Methods and results
[0062] 1 Preparation of Y187 competent yeast and transformation of recombinant bait plasmid pGBKT7-TRBD
[0063] 1.1 Streak the cryopreserved Y187 cell plate and culture in a 30°C incubator for 3-4 days until a single colony with a diameter of 2-3 mm grows;
[0064] 1.2 Pick Y187 yeast monoclonal cells, put them into a 50mL Erlenmeyer flask containing 10mL YPDA liquid medium, and blow evenly, 30°C, 210r / min shaking culture to OD 600 >1.5; take 4~5mL of yeast into 50mL YPDA liquid medium, culture at 30°C, shake at 210r / min until OD 600 =0.4~0.6;
[0065] 1.3 Collect the yeast liquid in a 50mL centrifuge tube, centrifuge at 700g for...
Embodiment 3
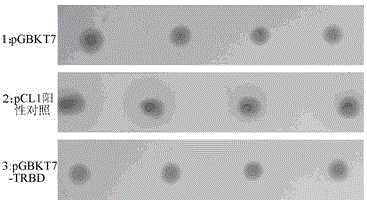
[0083] Screening of TRBD Interacting Proteins
[0084] 1. Materials
[0085] -Trp / -His / -Leu DO Supplement, -Trp / -His / -Leu / -Ade DO Supplement (Clontech).
[0086] 2. Methods and results
[0087] 1 Screening of proteins interacting with TRBD in yeast two-hybrid cDNA library
[0088] 1.1 Pick the Y187 monoclonal transformed into pGBKT7-TRBD in 50mL SD / -Trp liquid medium;
[0089] 1.2 30°C, 270r / min shaker culture to OD 600 >0.8 (16~20h), centrifuge at room temperature at 700g for 5min, discard the supernatant;
[0090] 1.3 Resuspend the bottom and count with a cell counting plate to adjust the cell concentration to ≥1×10 8 cells / mL;
[0091] 1.4 Mix 5mL bait bacteria Y187 and 1mL library AH109 cells (≥2×10 7 cells) in a 1L sterile Erlenmeyer flask;
[0092] 1.5 Add 45mL 2×YPDA liquid medium (containing Kan + 50μg / mL), the library tube was washed twice with 1mL 2×YPDA, and the washing solution was added to the Erlenmeyer flask;
[0093] 1.6 30 ℃ constant temperature s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com