Cefoperazone sodium tazobactam sodium medicine composition for injection and preparing process of cefoperazone sodium tazobactam sodium medicine composition
A technology of cefoperazone sodium and tazobactam sodium is applied in the directions of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, antibacterial drugs, etc. Oxidation and other problems, to reduce the concentration, reduce the risk of allergic reactions, reduce the effect of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
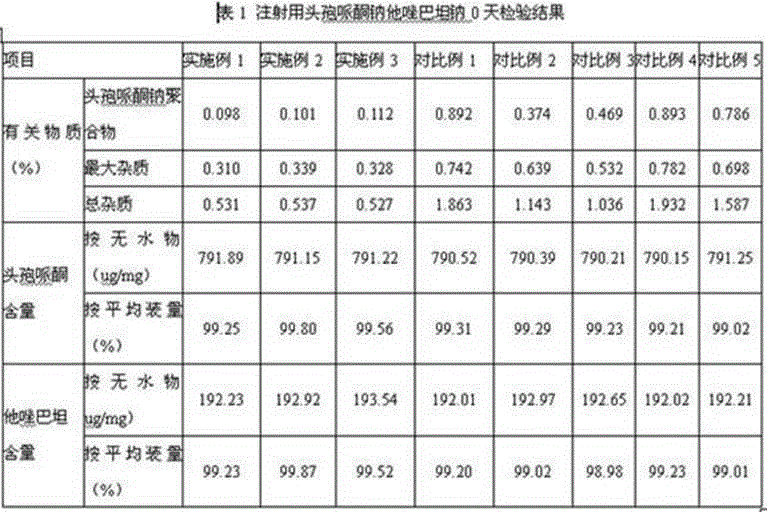
Embodiment 1
[0036] 1. Prescription (1000 bottles):
[0037] Cefoperazone 800g
[0038]Tazobactam 200 g
[0039] Sodium bicarbonate 152g.
[0040] 2. Preparation:
[0041] ① Take an appropriate amount of water for injection, cool it down to 6°C, and ultrasonically keep the dissolved oxygen in the water for injection below 2ppm. Accurately weigh 800g of cefoperazone and add it to the cooled water for injection, stir, and add 98.8g of sodium bicarbonate Salt formation, intermittent ultrasound, while detecting the pH of the solution, the pH is 5.5;
[0042] ②Measure an appropriate amount of water for injection, cool it down to 6°C, and use ultrasound to keep the dissolved oxygen in the water for injection below 2ppm. Accurately weigh 200g of tazobactam and add it to the cooled water for injection, stir, and add 53.2g of bicarbonate Sodium into salt, intermittent ultrasound, while detecting the pH of the solution, the pH is 5.7;
[0043] ③Mix the solution obtained in step ① and step ② eve...
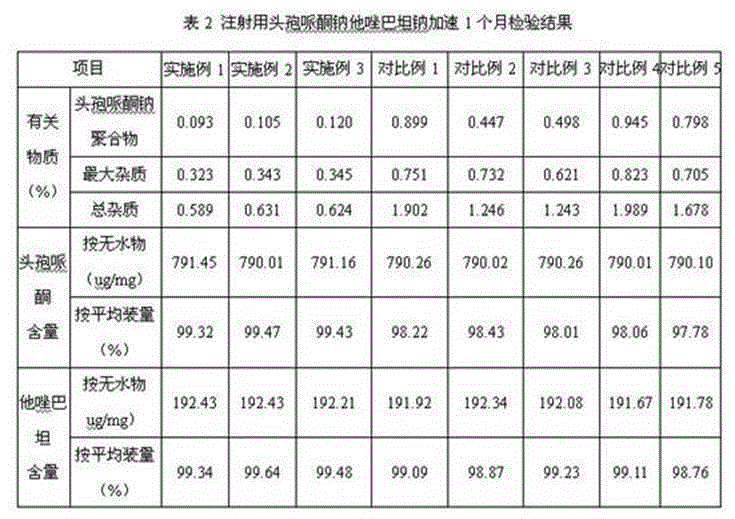
Embodiment 2
[0050] 1. Prescription (1000 bottles):
[0051] Cefoperazone 800g
[0052] Tazobactam 200 g
[0053] Sodium bicarbonate 160g.
[0054] 2. Preparation:
[0055] ① Take an appropriate amount of water for injection, cool it down to 6°C, and ultrasonically keep the dissolved oxygen in the water for injection below 2ppm. Accurately weigh 800g of cefoperazone and add it to the cooled water for injection, stir, and add 104 g of sodium bicarbonate Salt formation, intermittent ultrasound, while detecting the pH of the solution, the pH is 5.8;
[0056] ② Take an appropriate amount of water for injection, cool it down to 6°C, and ultrasonically keep the dissolved oxygen in the water for injection below 2ppm. Accurately weigh 200g of tazobactam and add it to the cooled water for injection, stir, and add 56g of sodium bicarbonate Salt formation, intermittent ultrasound, while detecting the pH of the solution, the pH is 6.0;
[0057] ③Mix the solution obtained in step ① and step ② even...
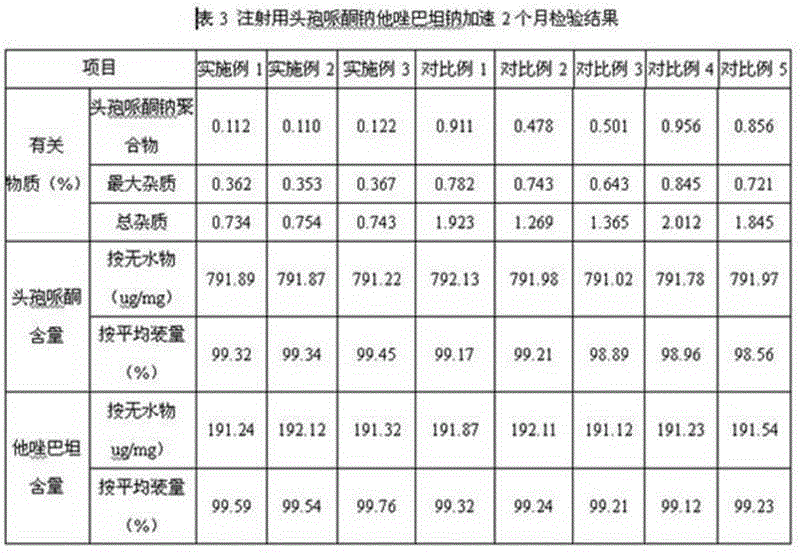
Embodiment 3
[0064] 1. Prescription (1000 bottles):
[0065] Cefoperazone 800g
[0066] Tazobactam 200 g
[0067] Sodium bicarbonate 168g.
[0068] 2. Preparation:
[0069] ① Take an appropriate amount of water for injection, cool it down to 6°C, and ultrasonically keep the dissolved oxygen in the water for injection below 2ppm. Accurately weigh 800g of cefoperazone and add it to the cooled water for injection. Stir, add 109.2g of sodium bicarbonate Salt formation, intermittent ultrasound, while detecting the pH of the solution, the pH is 6.0;
[0070] ②Measure an appropriate amount of water for injection, cool it down to 6°C, and use ultrasound to keep the dissolved oxygen in the water for injection below 2ppm. Accurately weigh 200g of tazobactam and add it to the cooled water for injection, stir, and add 58.8g of bicarbonate Sodium into salt, intermittent ultrasound, while detecting the pH of the solution, the pH is 6.3;
[0071] ③Mix the solution obtained in step ① and step ② evenl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com