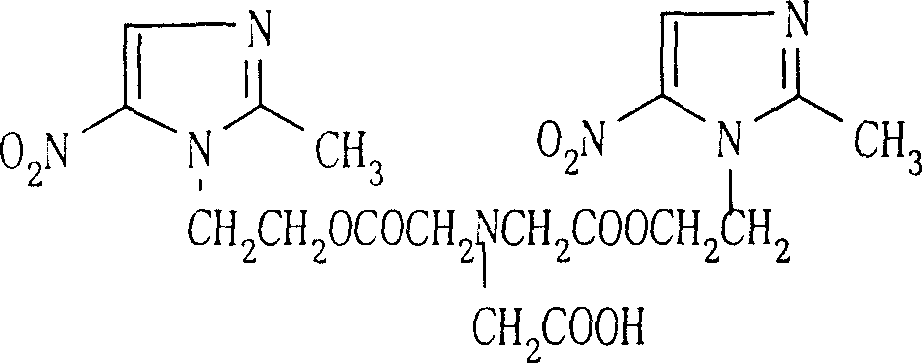Patents
Literature
31 results about "Bicarbonates sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
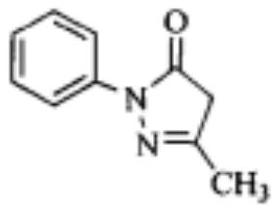
Metronidazole effervescence patch and technique of preparing the same
ActiveCN101152176AImprove the bactericidal effectGood synergyOrganic active ingredientsAntimycoticsChlorhexidine AcetateTalc
The invention discloses a Metronidazole, Clotrimazole and Chlorhexidine Acetate Effervescent Tablet and the preparation method. Each tablet has the following proportion of contents: metronidazole 0.2 gram, clotrimazole 0.16 gram, acetic acid chlorhexidine acetate 0.008 gram, fumaric acid 0.108 to 0.162 gram, tartaric acid 0.012 to 0.018 gram, solium bicarbonate 0.12 to 0.18 gram, starch 0.02 to 0.04 gram, dextrin 0.003 to 0.007 gram, hydroxypropyl cellulose 0.007 to 0.013 gram, talc 0.01 to 0.017 gram, sodium dodecyl sulfate 0.01to 0.02 gram, and proper proportion of povidone K30 water solution. According to the preparation method, the components are mixed to produce granule (1) and granule (2); the talc, sodium dodecyl sulfate and the granule (1) and granule (2) are mixed uniformly; granule content is tested and according to the granule content, the tablet weight is identified for tabletting. The invention overcomes the shortages with prior art, enables the acetic acid chlorhexidine acetate to perform functions normally, performs synergistic effects of the three drugs, and greatly enhances the cure rate. Besides, the frothing volume is high, and within storage period, the quality is stable and the drug effects are not affected.
Owner:SHANDONG SBOND PHARMA
Asymmetric syntheses method of (-)-sodium danshensu and application thereof
ActiveCN104744242ARaw materials are easy to getEasy to operateOrganic active ingredientsOrganic compound preparationSodium bicarbonateSynthesis methods
The invention discloses an asymmetric syntheses method of (-)-sodium danshensu and an application thereof, which belongs to the medicine chemical field. The method comprises the following steps: A)taking levodopa shown in a formula I as a raw material, and performing a hydroxyl acetylation to obtain S-3-(3,4-acetylphenyl)-2-alanine hydrochloride; B)performing a diazotization reaction on S-3-(3,4-acetylphenyl)-2-alanine hydrochloride obtained in the step A) to obtain S-3-(3,4-acetylphenyl)-2-hydracrylicacid in a formula III; C)performing hydrolysis on the S-3-(3,4-acetylphenyl)-2-hydracrylicacid in the step B to obtain S-3-(3,4-dihydroxy phenyl)-2-hydracrylicacid shown in a formula IV; and D)dropping a sodium bicarbonate saturated solution in the S-3-(3,4-dihydroxy phenyl)-2-hydracrylicacid to react to obtain sodium salt (-)-sodium danshensu shown in a formula V. The method has the advantages of easily available raw material and simple operation, and the (-)-sodium danshensu has good effect for treating myocardial ischemia and myocardial infarction.
Owner:HEBEI YILING MEDICINE INST
Operation rinsing water solution as well as preparation method and application thereof
InactiveCN104288176AImprove stabilityAntibacterial agentsHydroxy compound active ingredientsSodium bicarbonateSodium acetate
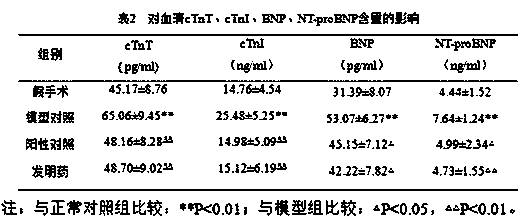
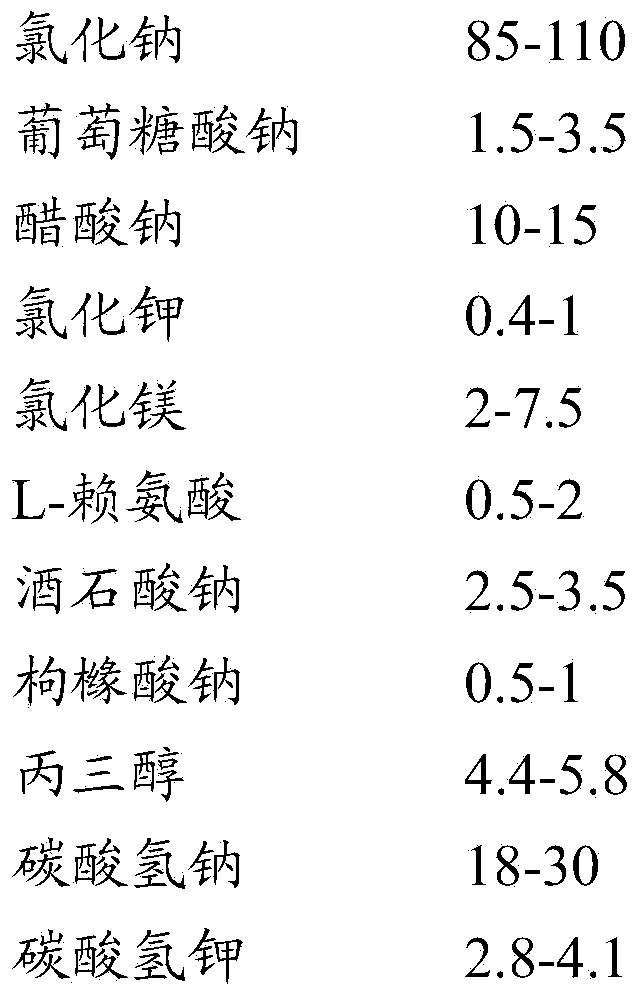
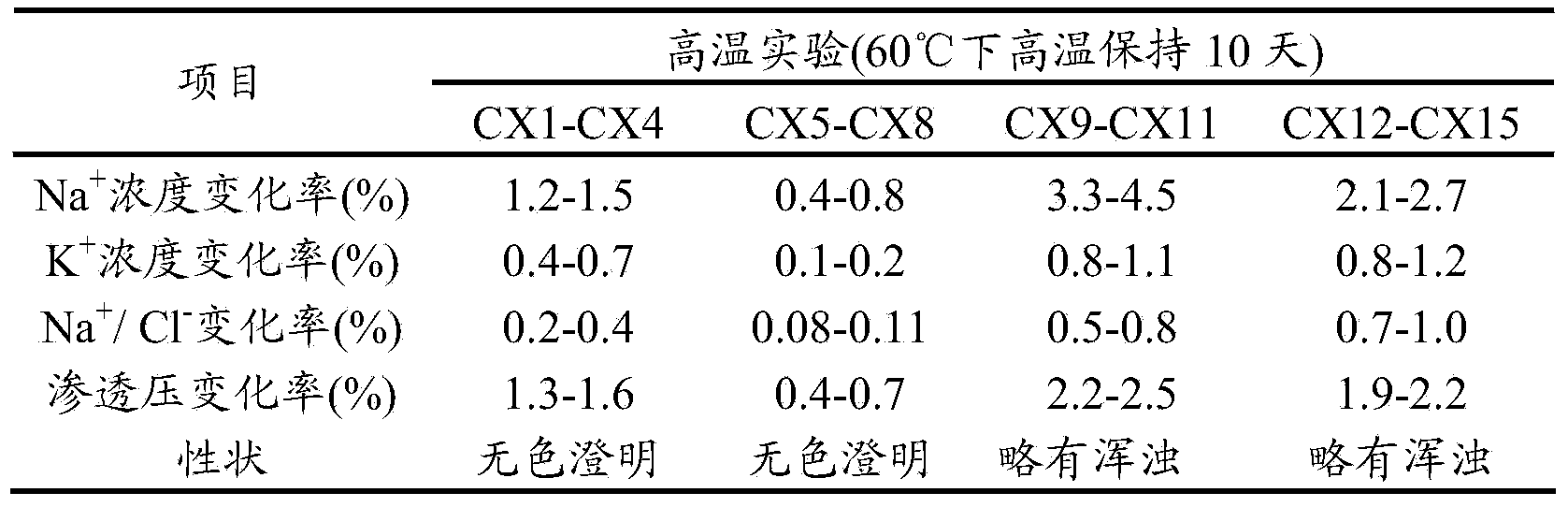
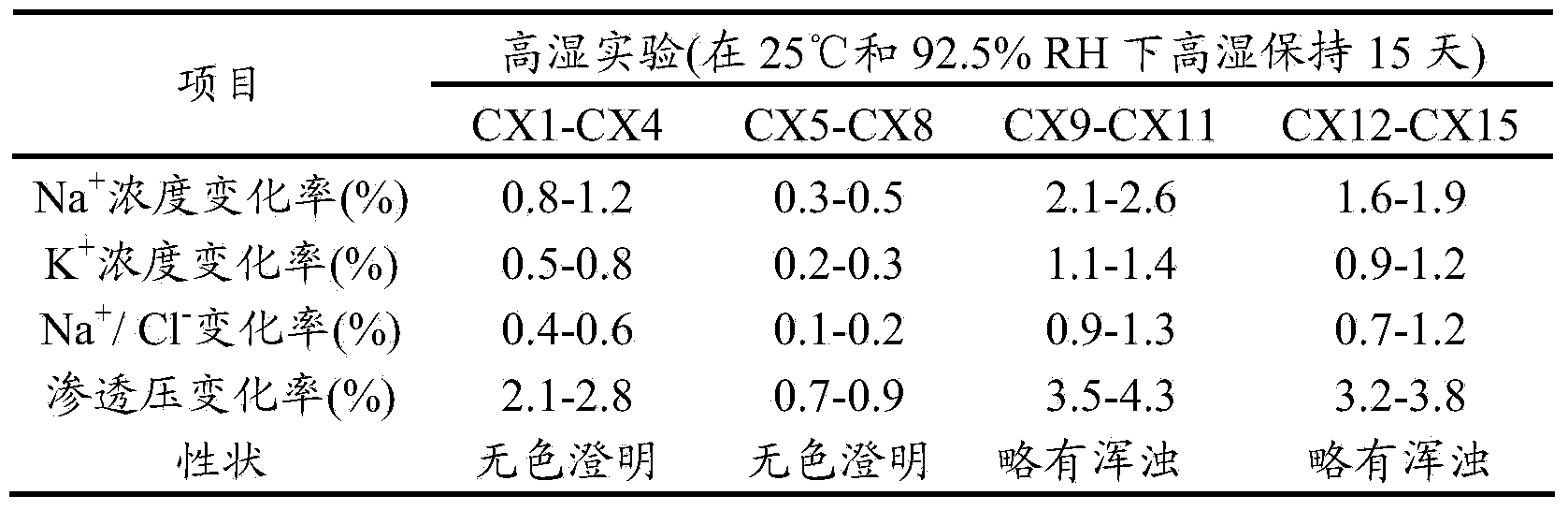
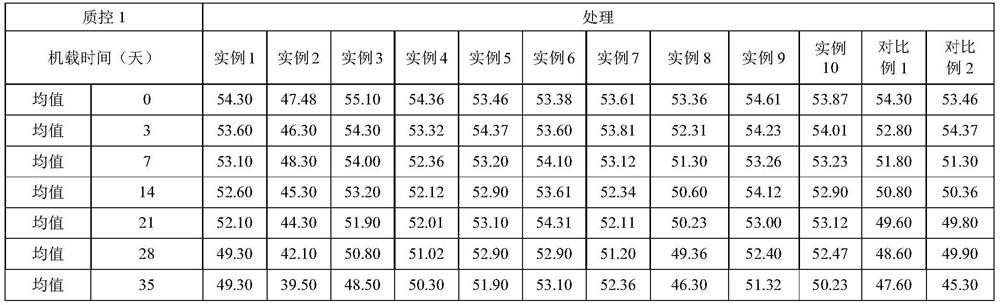
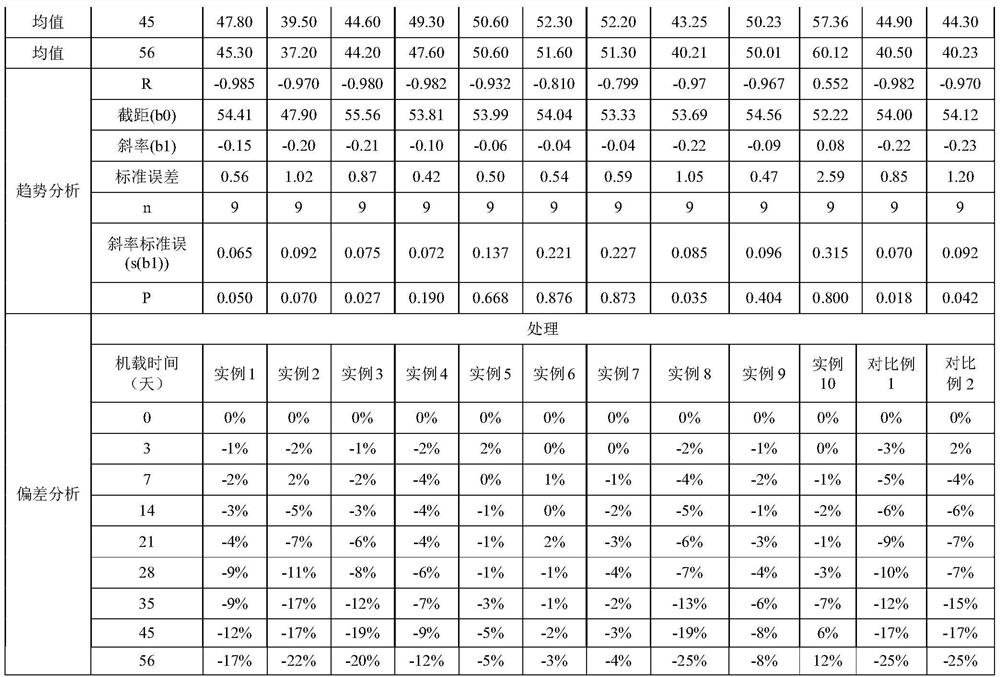
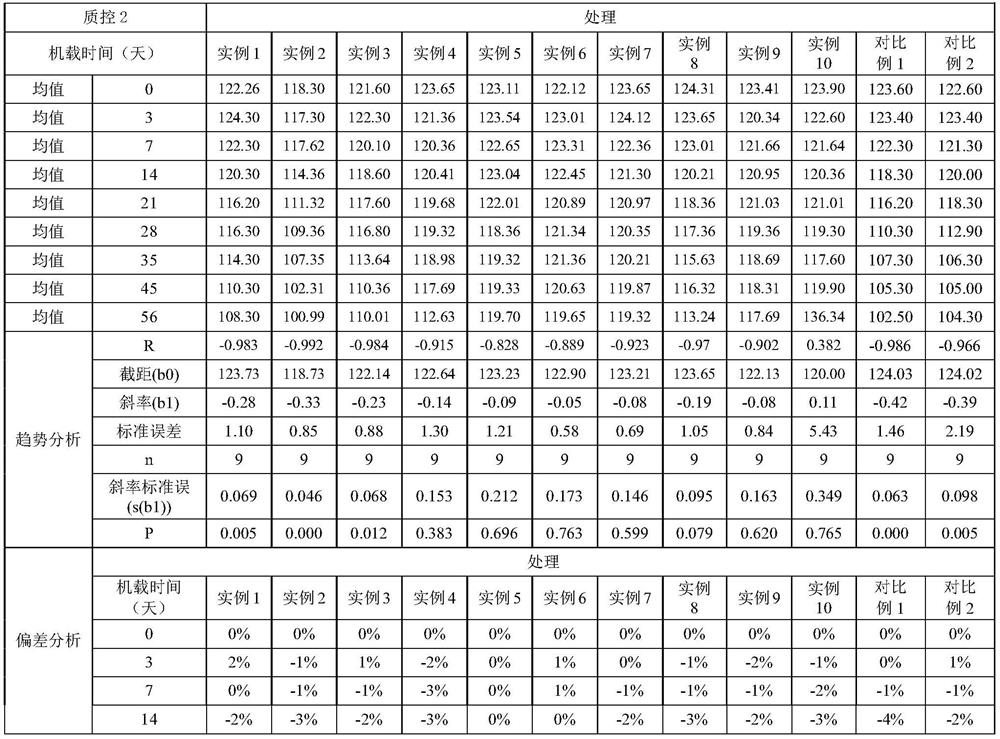
The invention relates to an operation rinsing water solution as well as a preparation method and application thereof. The rinsing water solution is prepared from sodium chloride, sodium gluconate, sodium acetate, potassium chloride, magnesium chloride, L-lysine, sodium tartrate, sodium citrate, glycerol, sodium bicarbonate and potassium bicarbonate. The operation rinsing water solution of which the sodium ion concentration, potassium ion concentration, Na<+1> / Cl<-1>, osmotic pressure and pH value are in specific ranges is prepared by specifically selecting specific ingredients properly determining usage and regulating pH value, and when the conditions are met at the same time, the operation rinsing water solution is safe and non-toxic, has excellent stability, and has tremendous application value and industrial production potential in medical field and clinical application.
Owner:ZHEJIANG TIANRUI PHARMA
Glutathione reductase detection kit as well as preparation method and application thereof
PendingCN113528609AReduce descent speedStable pH environmentMicrobiological testing/measurementActive agentPotassium ferricyanide
The invention provides a glutathione reductase detection kit and a preparation method and application thereof. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a potassium phosphate buffer solution, EDTA, GSSG, ascorbic acid oxidase, bilirubin oxidase, potassium ferricyanide, a surfactant, a first bacteriostatic agent and a second bacteriostatic agent; and the reagent R2 comprises a sodium bicarbonate buffer solution, NADPH, the first bacteriostatic agent, a surfactant and a stabilizer. Compared with the prior art, the glutathione reductase detection kit provided by the invention has the advantages of good airborne stability, high sensitivity and strong antibacterial ability, and can meet requirements of clinical application of glutathione reductase detection.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Preparation method of low-odor foamed polypropylene material
InactiveCN110698765APromote environmental protectionNo effect on mechanical propertiesAlcoholCyclohexanol
The invention relates to a preparation method of a low-odor foamed polypropylene material, and the method comprises the following steps of: weighing polypropylene resin, a sodium borohydride alcohol solution and auxiliary materials, and mixing for at least 1h under the condition that the temperature is not lower than 100 DEG C; then melting, extruding, cooling and setting; after that, mixing withsodium bicarbonate and performing injection molded. The method introduces a sodium borohydride cyclohexanol solution; sodium borohydride can reduce formaldehyde to methanol, cyclohexanol can dissolveformaldehyde and other harmful organic substances and bring them out when vaporized; therefore, the content of the formaldehyde and other harmful organic substances in the material can be effectivelyreduced, the pungent smell of the material and the amount of the harmful substances the can be volatilized are reduced, and the environmental protection of the polypropylene material is improved; at the same time, the mechanical properties test results show that the introduction of the sodium borohydride cyclohexanol solution has no effect on the overall mechanical properties of the material.
Owner:SUZHOU RUNJIA ENGINEER PLASTIC
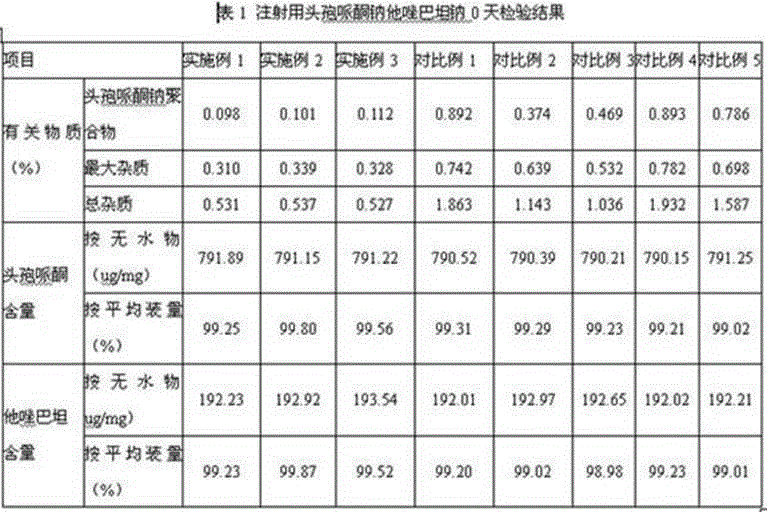
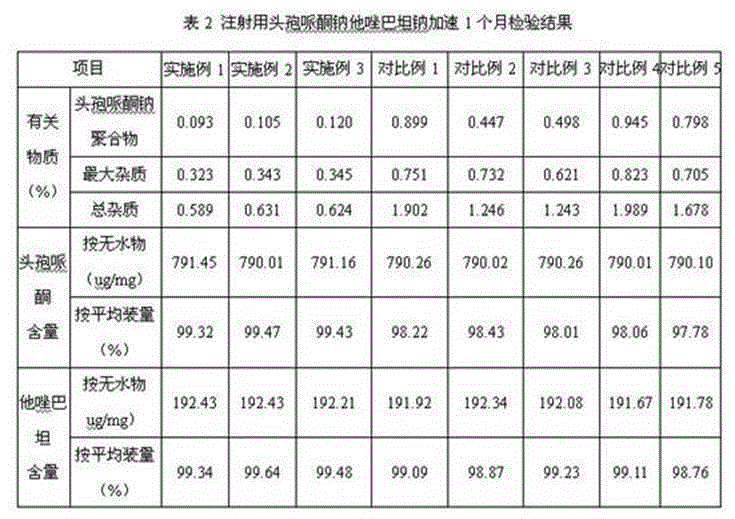
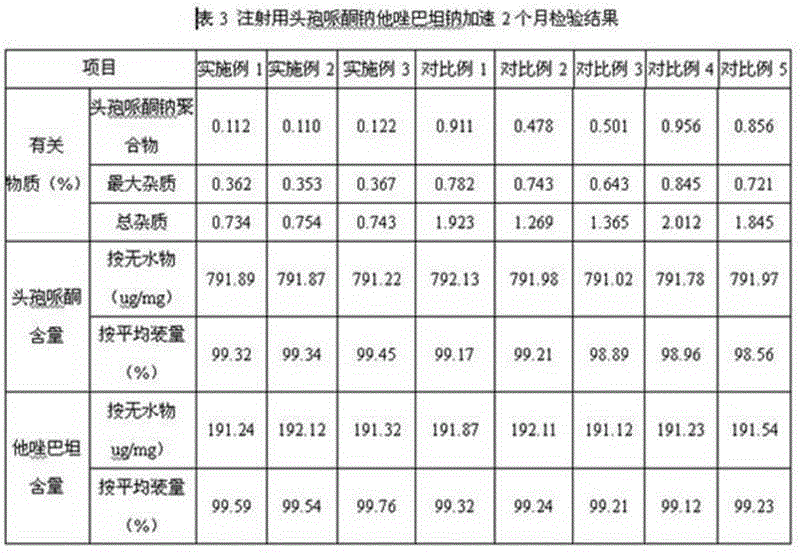
Cefoperazone sodium tazobactam sodium medicine composition for injection and preparing process of cefoperazone sodium tazobactam sodium medicine composition
ActiveCN104958302AReduce generationAvoid it happening againAntibacterial agentsHeterocyclic compound active ingredientsDrugs preparationsAnaphylactic reactions
The invention relates to the field of medicine preparations, and in particular to a cefoperazone sodium tazobactam sodium medicine composition for injection and a preparing process of the cefoperazone sodium tazobactam sodium medicine composition. The cefoperazone sodium tazobactam sodium medicine composition for injection comprises cefoperazone, tazobactam and sodium bicarbonate, and the weight ratio of the cefoperazone to the tazobactam to the sodium bicarbonate is 8:2:1.52-1.68. By means of the medicine composition, the clinic safety performance is improved, and risks of anaphylactic reactions are reduced; and meanwhile the product quality is guaranteed. The invention further provides the preparing process of the medicine composition. The process is simple, the cost is saved, the mixing uniformity of the medicine composition can be effectively improved, generation of polymer in the preparing process is effectively reduced, the polymer and other impurities generated by the high temperature in the split packaging process are prevented, and meanwhile oxidation of active ingredients in the preparing process is reduced.
Owner:JINAN KANGHE MEDICAL TECH
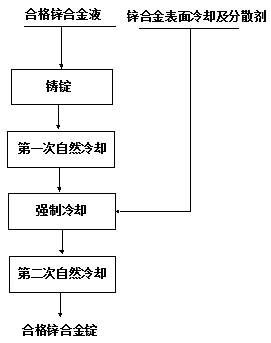
A cooling liquid for alloy ingot
ActiveCN110628394BDoes not affect cooling rateAvoid sudden temperature changesHeat-exchange elementsCelluloseZinc alloys
The invention relates to an alloy ingot cooling liquid, which belongs to the technical field of alloy casting. The invention comprises 980-998 parts of water, 1.2-12 parts of sodium alkylbenzene sulfonate, 0.2-2 parts of sodium sulfate, and 0.2-2 parts of sodium tripolyphosphate 2 parts, 0.2-2 parts of carboxymethyl cellulose, 0.1-1 part of acrylic acid, 0.1-1 part of sodium bicarbonate; the alloy ingot cooling liquid of the present invention can rapidly cool the zinc alloy surface, and the zinc alloy will not crack , water ripples and bulges, it has the advantages of low cost, simple process, convenient operation and good environmental protection effect.
Owner:YUNNAN CHIHONG RESOURCE COMPREHENSIVE UTILIZATION CO LTD
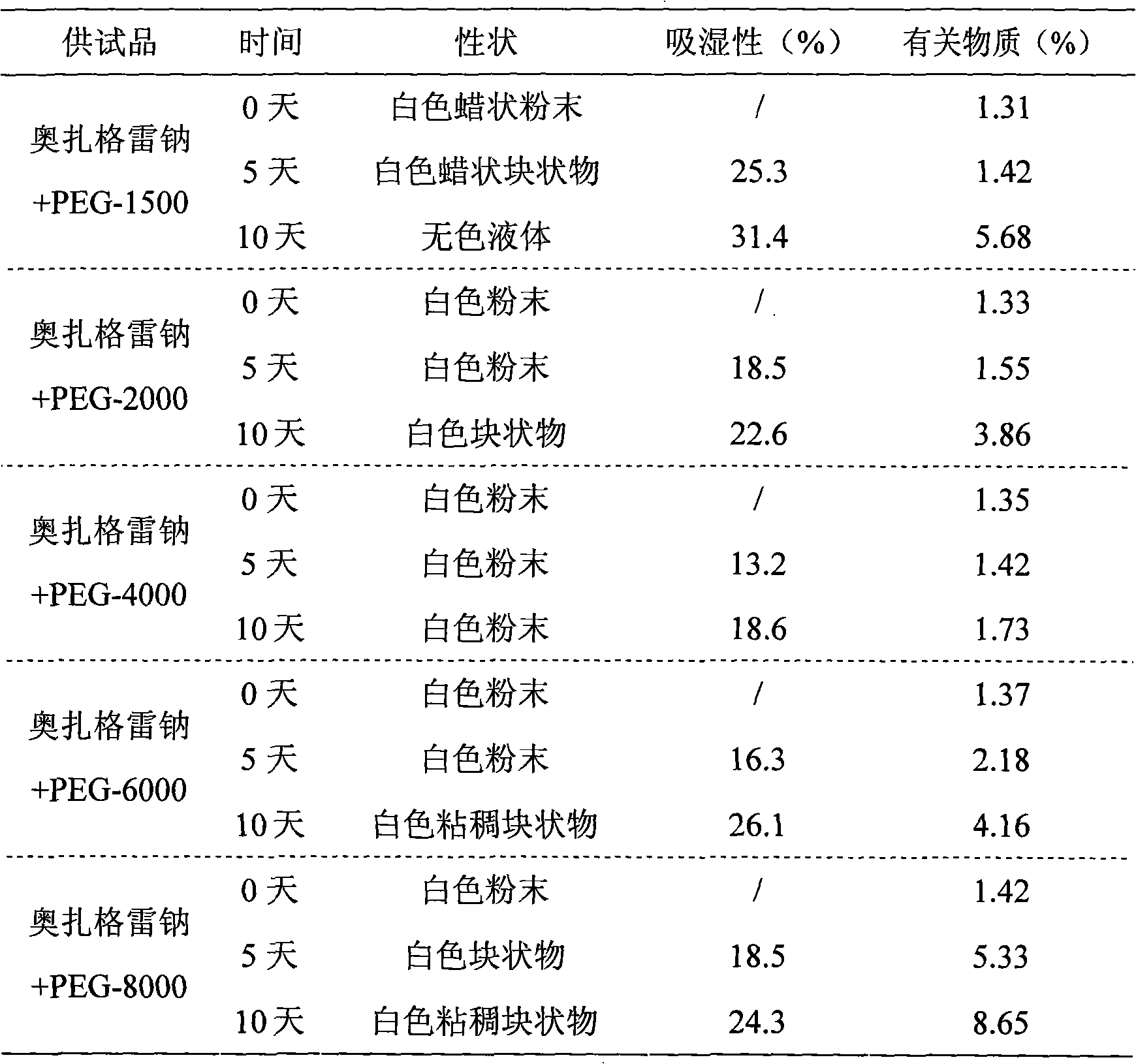
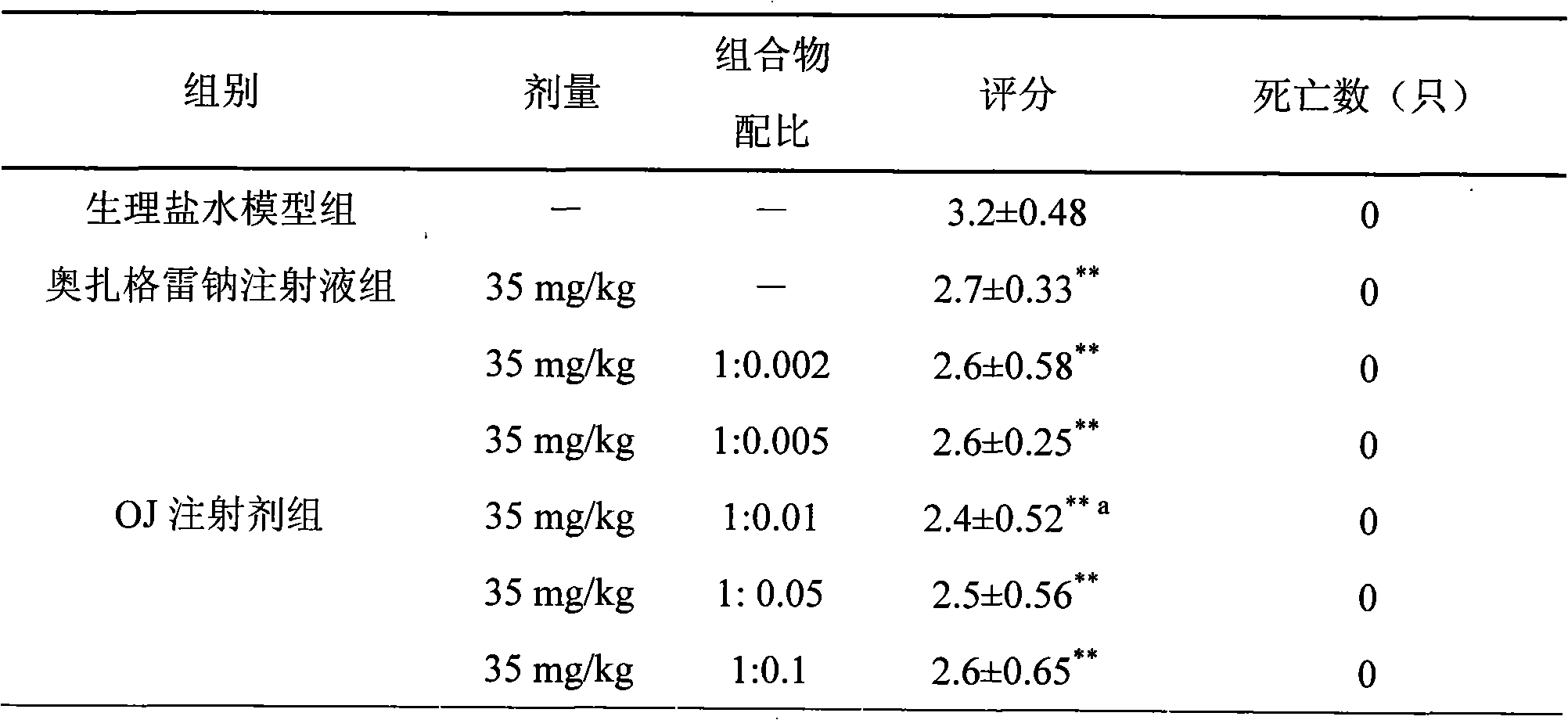
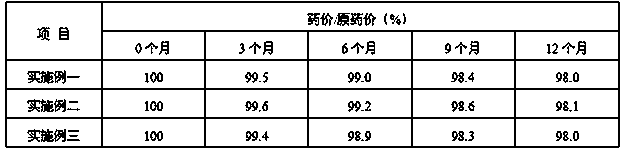
Composition of sodium ozagrel and polyethylene glycol and preparation method thereof
InactiveCN101780072AImprove effectivenessImprove in vivo stabilityOrganic active ingredientsPowder deliverySodium bicarbonateMANNITOL/SORBITOL
The invention discloses a composition of sodium ozagrel and polyethylene glycol and a preparation method thereof, belonging to the medical technical field. The weight ratio of sodium ozagrel to polyethylene glycol in the pharmaceutical composition is 1:0.002-0.1. Injections can be prepared by using the pharmaceutical composition and auxiliary material which is acceptable in pharmacy and the auxiliary material is selected from one or more of lactose, mannitol, sorbitol, dextran, citric acid-sodium citrate and sodium bicarbonate-sodium carbonate. The pharmaceutical composition is used to cure dyskinesia associated with acute thrombotic infarction and cerebral infarction.
Owner:BEIJING SIHUAN PHARMA +1
A kind of esomeprazole sodium sodium chloride injection and preparation method thereof
ActiveCN107773529BAvoid pollutionImprove stabilityOrganic active ingredientsDigestive systemDisodium EdetateSodium Chloride Injection
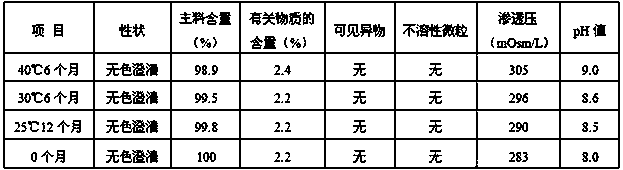
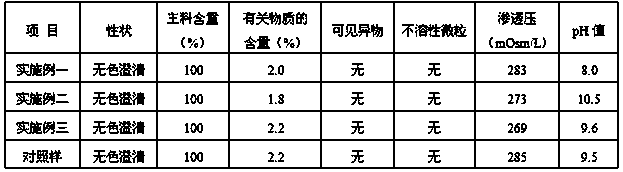
The invention discloses an esomeprazole sodium and sodium chloride injection and a preparation method thereof, and belongs to the technical field of an injection. Every 100 mL of the injection includes: 35-45 mg of esomeprazole sodium, 1.5-4.5 mg of edetate disodium, 6-10 mg of sodium hydroxide, 4-8 mg of sodium bicarbonate, 0.9 g of sodium chloride, and a proper amount of injection water. The invention further provides a preparation method of the injection. Through prescription optimization, sodium bicarbonate, sodium chloride and injection water are added in the prescription, wherein sodiumbicarbonate has an effect of buffering and adjusting the pH value and makes the pH value of the injection always maintain in an effective pH value range of a medicine. The injection can be directly used, so that pollution during a preparation process is prevented. The injection is good in stability and not prone to degrade. According to an accelerated test, the injection can be stored for 6 monthswith the efficacy only reduced by 5.5%. The injection can be stored for 12 months at the room temperature with the efficacy being 98% of the original efficacy, and meets the quality standard.
Owner:湖北华仁同济药业有限责任公司
A kind of high-efficiency dilution powder of pig fresh essence stored at 4 ℃ and preparation method and application
ActiveCN109169636BRich preservation technology meansLong storage timeDead animal preservationPig farmsAnimal science
Owner:天津市农业科学院
Meloxicam-Containing Granulated Product
PendingUS20220249506A1High dissolution rateOrganic active ingredientsNervous disorderMeloxicamArginine
To provide a technique for improving the dissolution of meloxicam. A granulated product, including the following components (A), (B), and (C): (A) meloxicam or a salt thereof, or a solvate thereof; (B) one or more basic compounds selected from the group consisting of potassium bicarbonate, sodium bicarbonate, arginine, lysine, magnesium hydroxide, and magnesium oxide; and (C) a water-swellable polymer.
Owner:SS PHARMA CO LTD
Compressed capsules for giving birth to males
ActiveUS20190183906A1Satisfies needEasy to swallowOrganic active ingredientsSexual disorderSodium bicarbonateBumetanide
The present invention is related to baby love male capsules including active ingredients such as fludrocortisone, bumetanide, sodium bicarbonates, sodium chloride, and Licourice extract, inactive ingredients such as gelatin, plasticizers, moisture absorbents, and preservatives.The active ingredients are selected according to ovum wall thickness and cell fragility theories. The present invention leads to a breakthrough in the medical field through which the baby gender can be determined and many chronic diseases can be cured.
Owner:RASHWAN EMAD ABD ELAZEEM
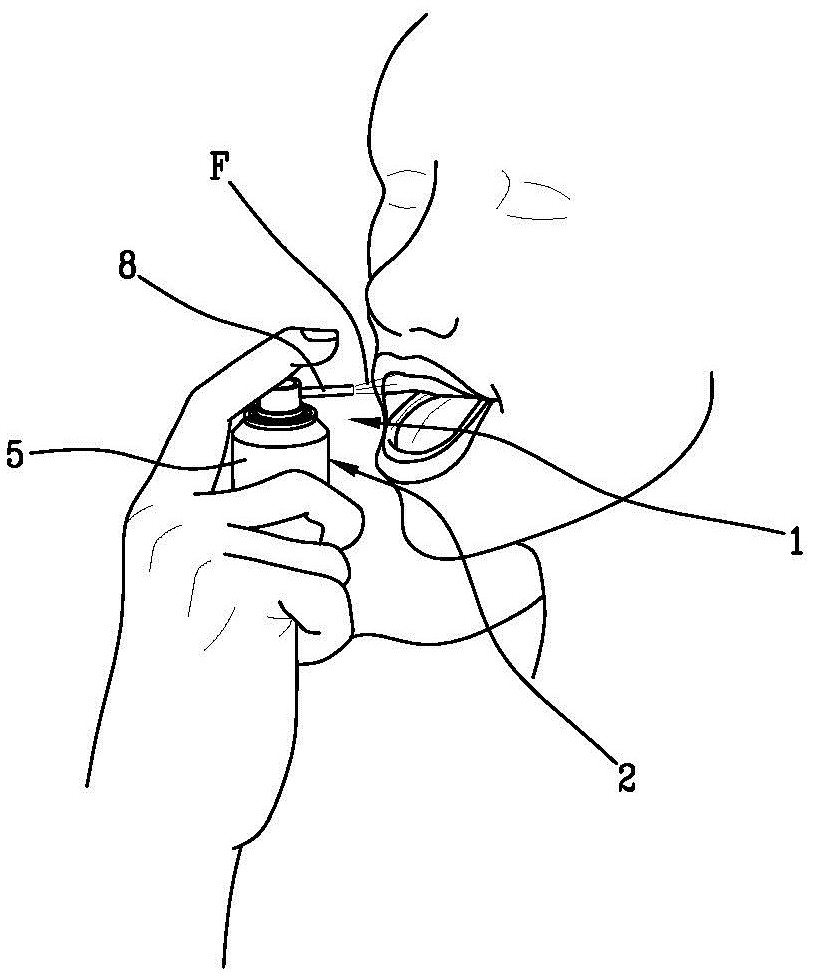
Compositions for use in dentistry and related devices for treatment of the oral cavity
ActiveCN108371564BEfficient removalIntegrity guaranteedAntibacterial agentsCosmetic preparationsOral treatmentLiquid state
A composition (C) for use in dentistry and a device (1) for treating the oral cavity, the device comprising: a housing chamber (2) containing the composition (C) in liquid state; a conduit (3) comprising For discharging a composition (C) configured to generate a flow (F) at a given discharge pressure; and a delivery valve (4) interposed between the holding chamber (2) and the discharge conduit (3) During the period, the valve (4) can be switched between a delivery state and a closed state. In the delivery state, the accommodating chamber (2) is fluidly connected with the conduit (3) to allow a predetermined amount of the composition (C) to pass through. In the closed state , the composition (C) is not allowed to pass between the holding chamber (2) and said duct (3). The composition comprises 10-15 wt% sodium bicarbonate and 100 wt% water.
Owner:32 LABS SRL
Peritoneal dialysis solution with high de-watering rate
InactiveCN107854482AOrganic active ingredientsPharmaceutical delivery mechanismSodium bicarbonatePotassium
The invention discloses a peritoneal dialysis solution with high de-watering rate and solves the de-watering problem of the dialysis solution. The peritoneal dialysis solution comprises 10-15 percentby W / V of sodium chloride, 0.2-5 percent by W / V of calcium chloride, 2-5 percent by W / V of potassium chloride, 2-5 percent by W / V of sodium bicarbonate, 2-5 percent by W / V of magnesium chloride, 2-10percent by W / V of monascus polysaccharide and 0-5 percent by W / V of maltose.
Owner:宋宏婷
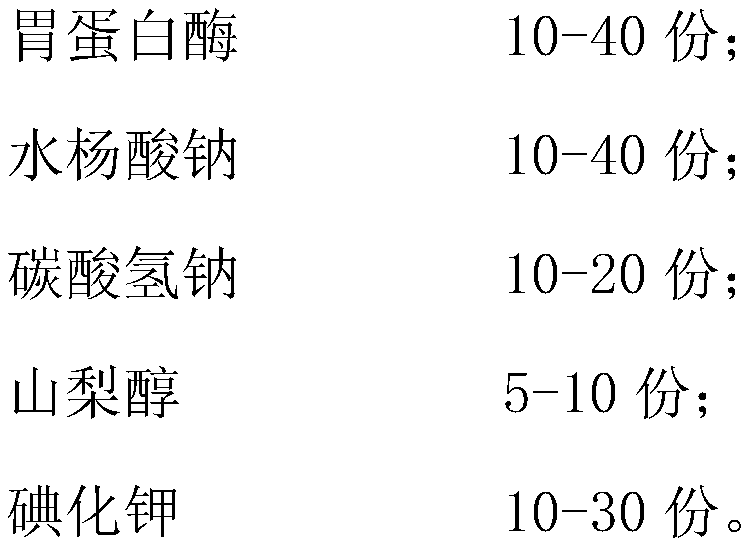
A composition for promoting coccidia oocyst value determination and its preparation method
ActiveCN106983858BIncreased ability to enter cellsAvoid damageSalicyclic acid active ingredientsPeptide/protein ingredientsBiotechnologyCoccidia
The invention relates to a composition for promoting the colonization performance of coccidian oocysts and a preparation method thereof. The composition comprises, by weight, 10 to 40 parts of pepsin, 10 to 40 parts of sodium salicylate, 10 to 20 parts of sodium bicarbonate, 5 to 10 parts of sorbitol and 10 to 30 parts of potassium iodide. The composition is scientific and reasonable in composition, simple in preparation process, convenient to use and low in cost; and the composition can rapidly accelerate excystation of sporulated oocysts to release sporozoites, allows the sporozoites to rapidly enter intestinal epithelial cells for schizogenesis and gamogenesis so as to reduce loss of the sporozoites with intestinal contents, improves bonding between the sporozoites and cells, realizes rapid colonization of the sporozoites and enhances the utilization rate of a coccidium vaccine.
Owner:TIANJIN HLINTE BIOTECH CO LTD
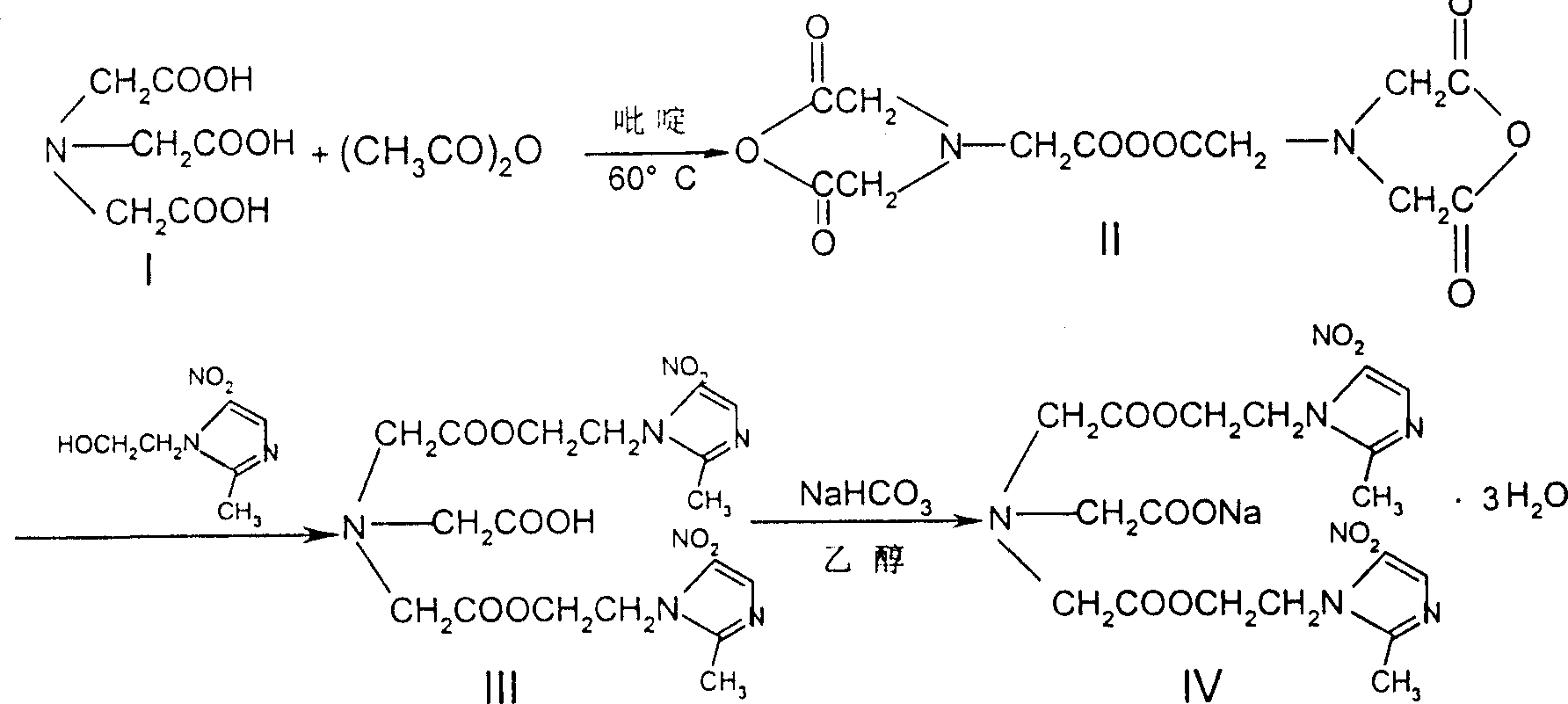
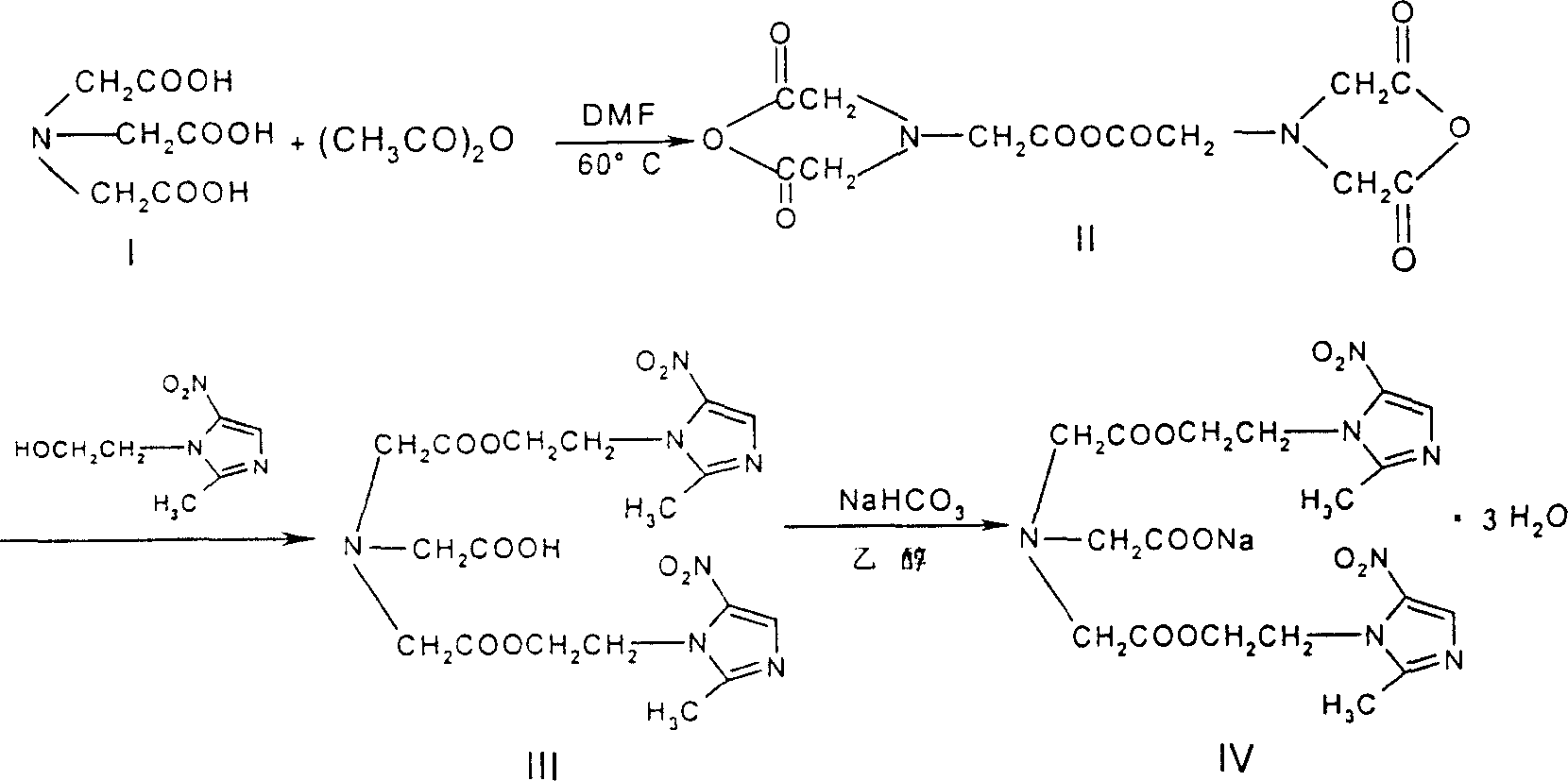
Process for synthesis of sodium glycididazole
InactiveCN100341858CHigh synthetic yieldEffective controlOrganic chemistrySodium bicarbonateAcetic acid
This invention belongs to medicine chemical technology field. This invention discloses a new synthesis technique of dry ammonia double azole natrium. The material is nitrilotriacetic acid, then it reacts with acetic anhydride in N, N-dimethyl amide, and product is got after refining and salifying with bicarbonates. The reaction yield is high, cost is low of this technique, labor intensity is reduced, and it is convenient to scale producing and environment protection.
Owner:SHANDONG LUYE PHARMA CO LTD
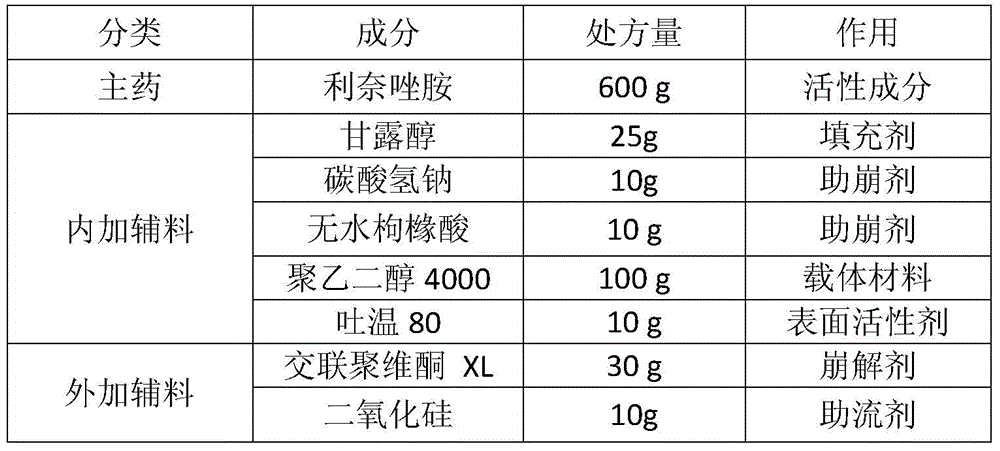
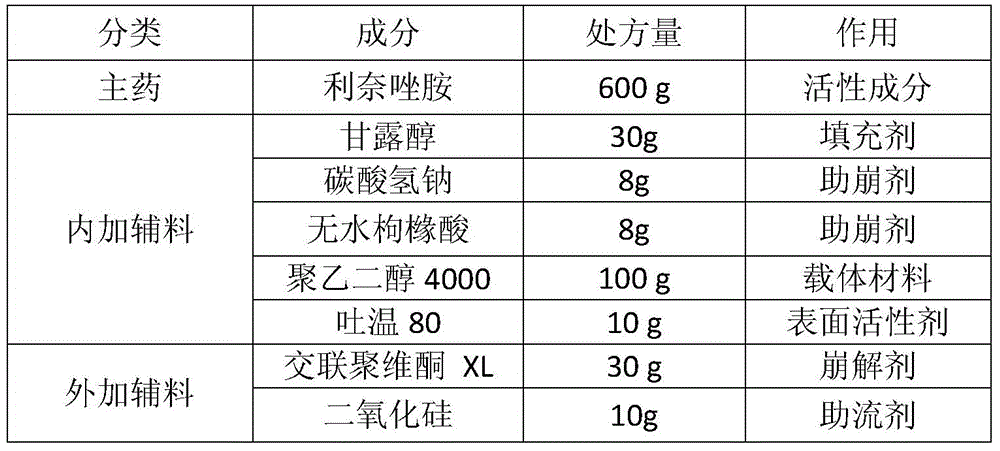
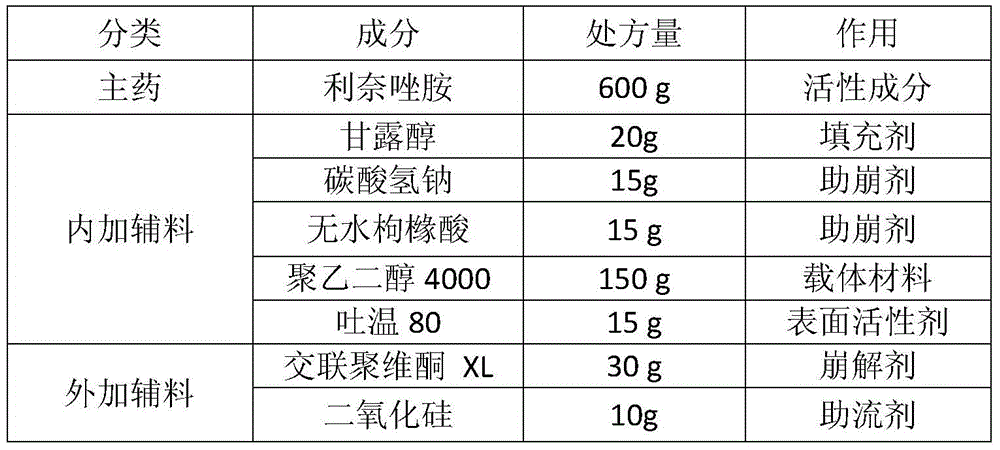
A composition containing linezolid and its preparation method
ActiveCN104173303BEasy to solveMeet the requirementsAntibacterial agentsOrganic active ingredientsPolyethylene glycolSolvent free
The invention relates to a composition containing linezolid tablets, which is composed of mannitol, sodium bicarbonate, anhydrous citric acid, polyethylene glycol, Tween 80, crospovidone and silicon dioxide; the invention also discloses A method for preparing a linezolid composition was developed. After solvent-free spray granulation and ethylene glycol surface modification, the technical defects of linezolid crystal form IV easy to change crystal form and produce impurities were solved during the preparation process. . The linezolid composition disclosed by the invention, especially the linezolid tablet, has stable quality and good dissolution rate; the disclosed preparation method is simple in operation and low in cost, and is very suitable for industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Ceftezole sodium powder injection and synthesizing method thereof
ActiveCN100506210CHigh purityImprove stabilityAntibacterial agentsPowder deliveryCeftezole SodiumReaction intermediate
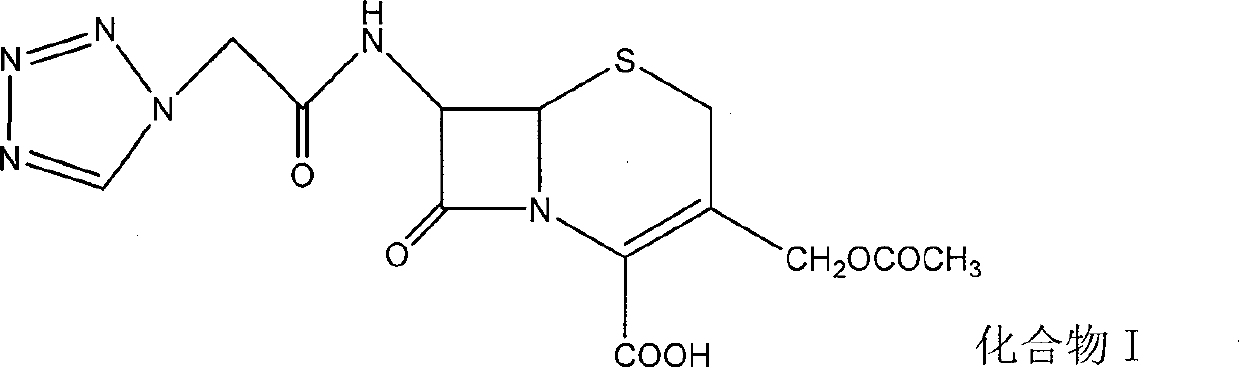
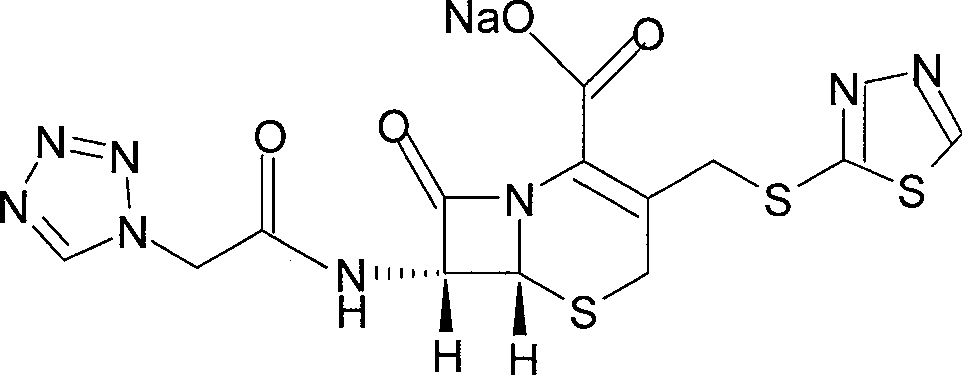
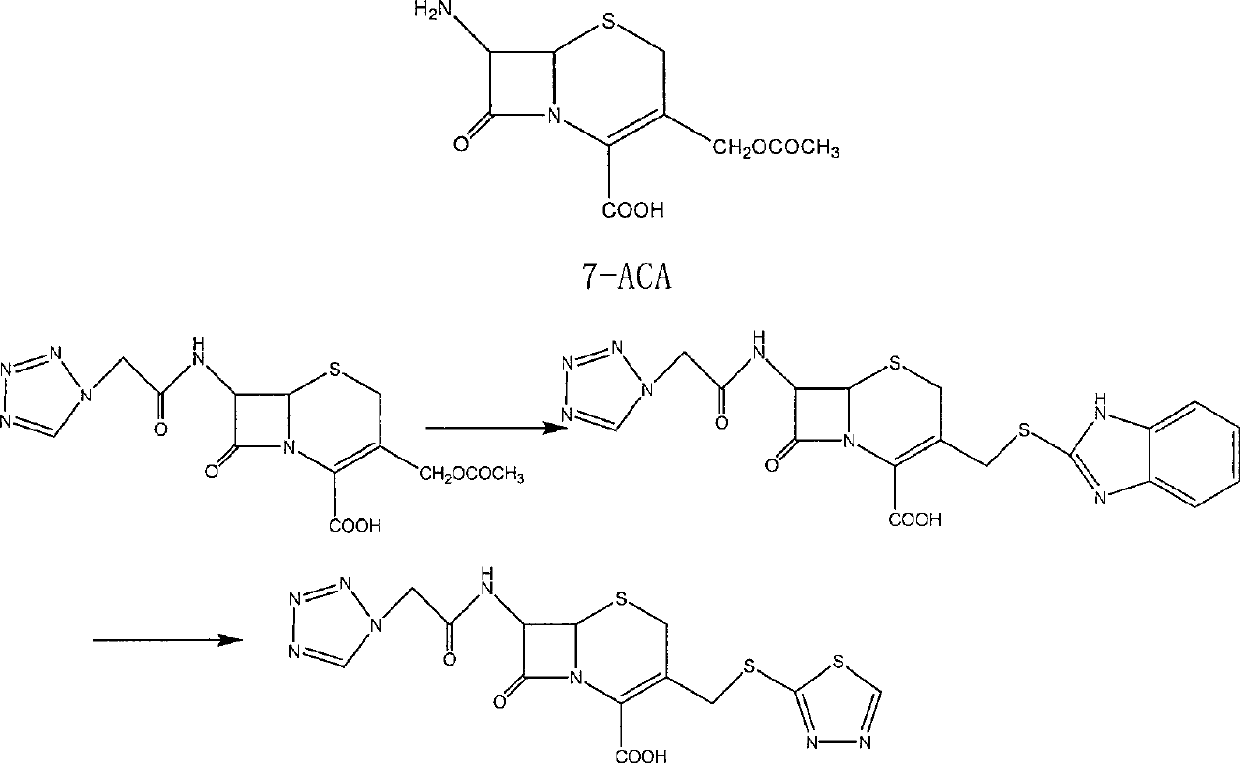
The invention provides a ceftezole sodium powder injection and a synthesis method thereof. The powder injection is composed of more stable ceftezole sodium crystals. The method reacts tetrazoleacetic acid, N, N'-dicyclohexylcarbodiimide and 7-aminocephalosporanic acid in a ratio of 1.4~1.7:1~1.3:1 in dimethyl sulfoxide, and then The reaction intermediate is reacted with 1.1-1.3 times of thiadiazole mercaptan to prepare ceftezole, and after recrystallization with acetone, ceftezole sodium is prepared in aqueous sodium bicarbonate solution. The method is simple and easy, and the total yield is as high as 55.5%.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
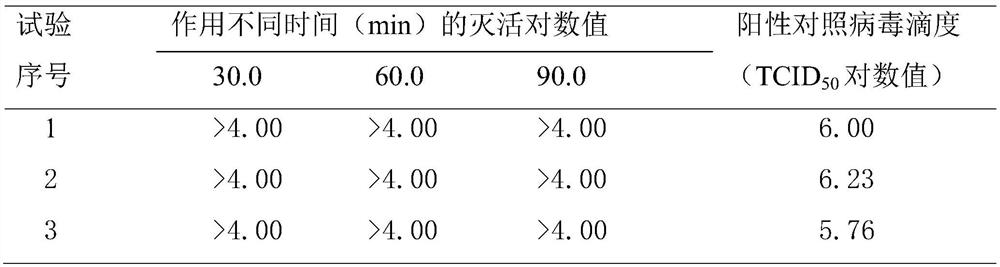
Novel application of sodium bicarbonate in prevention and treatment of human coronavirus infection
The invention relates to a novel application of sodium bicarbonate in prevention and treatment of human coronavirus infection. A sterilized aqueous solution is prepared from the sodium bicarbonate by using a conventional process and is used for preparing a spray which is sprayed into the mouth, nose and throat and is inhaled into the lung with breathing, an aerosol and an injection; the upper respiratory tract, including the oral cavity, is sprayed, the lower respiratory tract is administrated by atomization, the viral envelop lipidosome is broken by lipolysis, the coronavirus envelop is broken to inactivate the virus and destroy and block the viral envelop to combine with a receptor, so that the coronavirusis inactivated by losing the recognition and adsorption capacity of host cells. The application is used for controlling and treating coronavirus infection.
Owner:北京中卫神农慢性病医学研究院有限公司
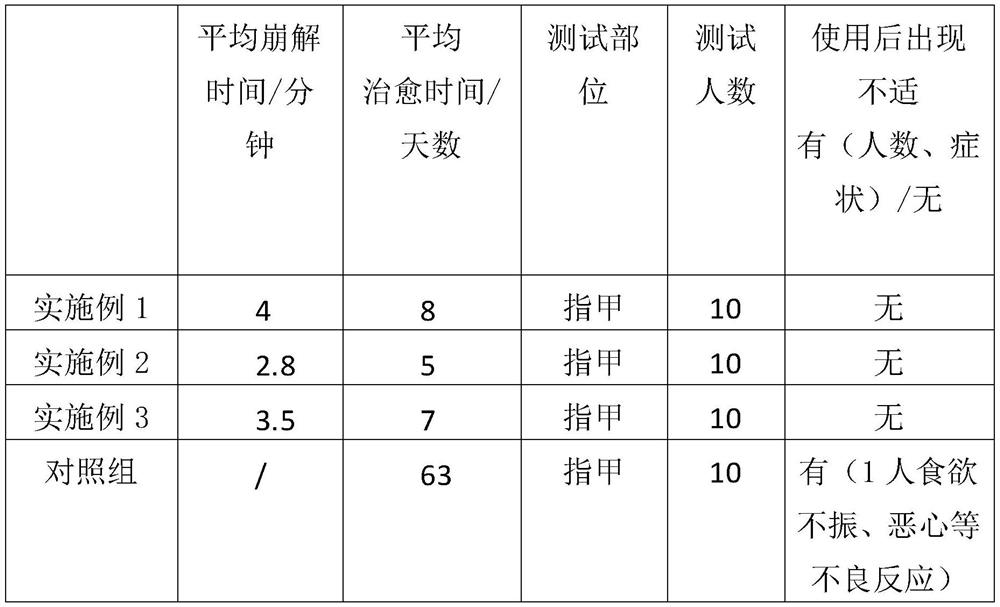
A kind of terbinafine hydrochloride effervescent tablet and using method thereof
ActiveCN112220763BPromote dissolutionAvoid churnOrganic active ingredientsAntimycoticsCelluloseAmytal Sodium
The invention discloses a terbinafine hydrochloride effervescent tablet, comprising 120-150 parts of terbinafine hydrochloride, 160-190 parts of citric acid, 160-190 parts of sodium bicarbonate, and 20-35 parts of sodium starch glycolate and 15-20 parts of low-substituted hydroxypropyl cellulose, the auxiliary materials include by weight: 285-315 parts of lactose, 20-30 parts of polysorbate 80, 35-55 parts of povidone K30, 70-90 parts of dehydrated alcohol parts and 8-12 parts magnesium stearate. In the present invention, by adding citric acid and sodium bicarbonate, the dissolution of the terbinafine hydrochloride effervescent tablet during use is accelerated. Method, use warm water to accelerate the dissolution of the product in water, stir evenly, and then soak the feet or hands containing the lesions in the liquid medicine. This method of use is targeted and prevents the loss of the medicine. The dosage can be adjusted according to the severity of the lesions, and the effect is fast. , high safety, small side effects, significant curative effect, short treatment time, and a wide range of applicable objects.
Owner:上海桓华制药有限公司
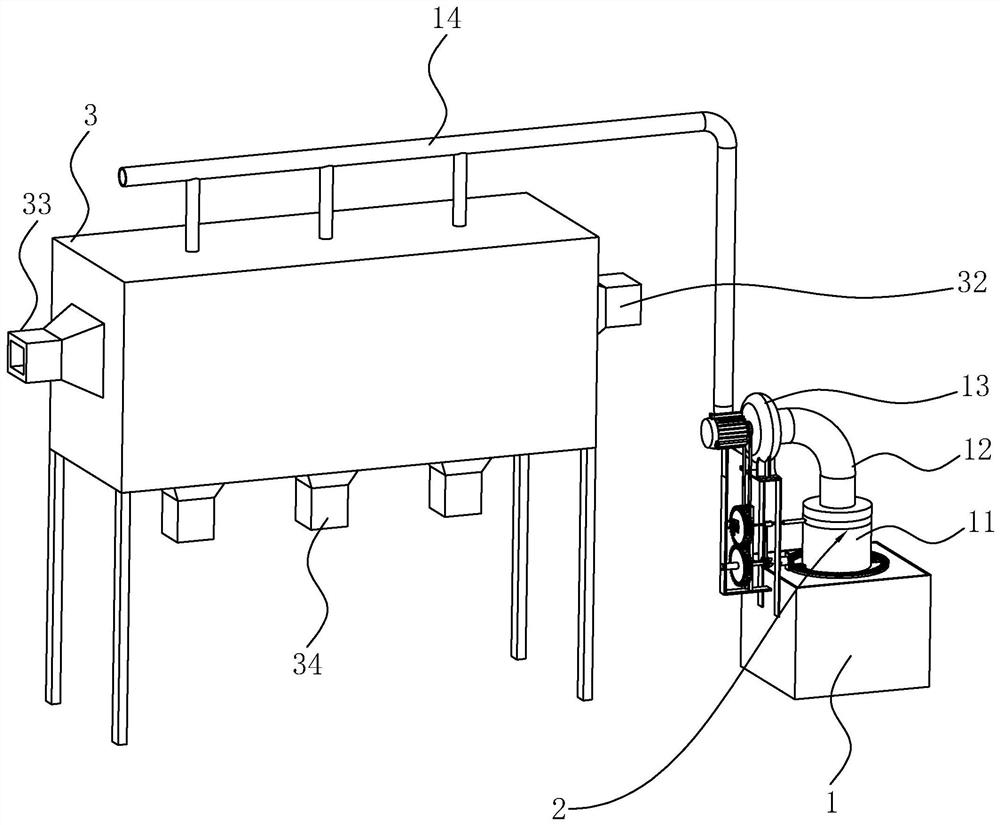
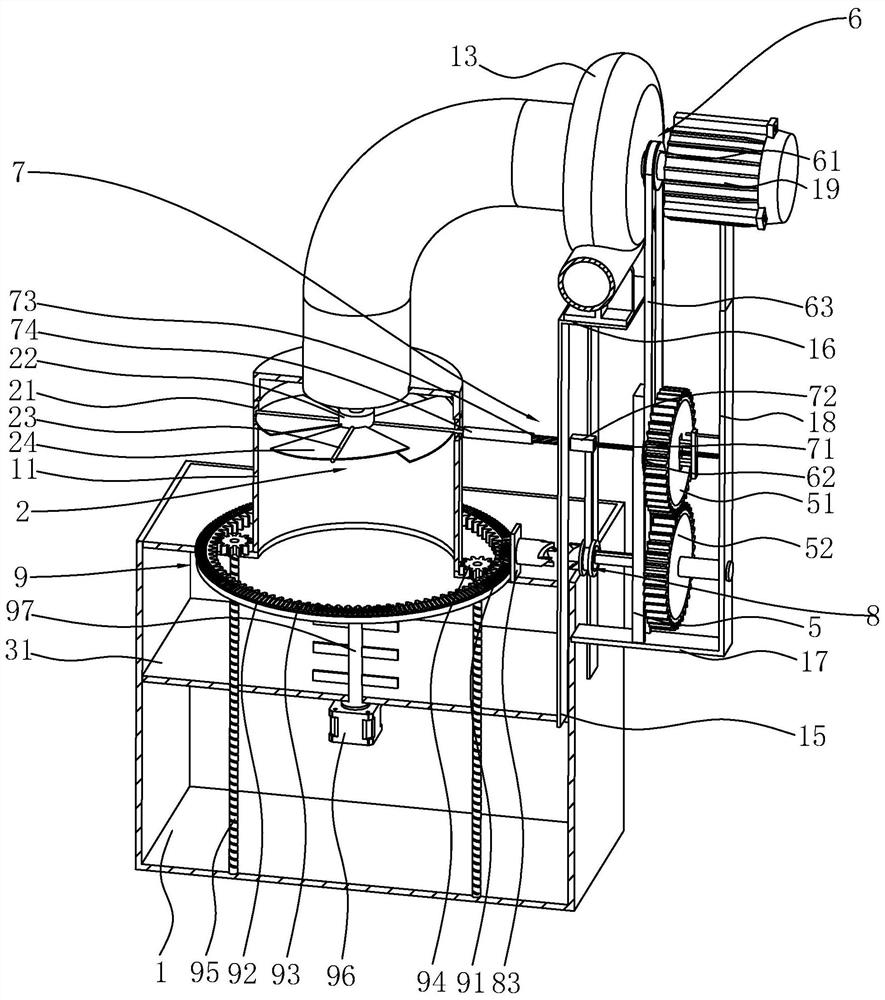
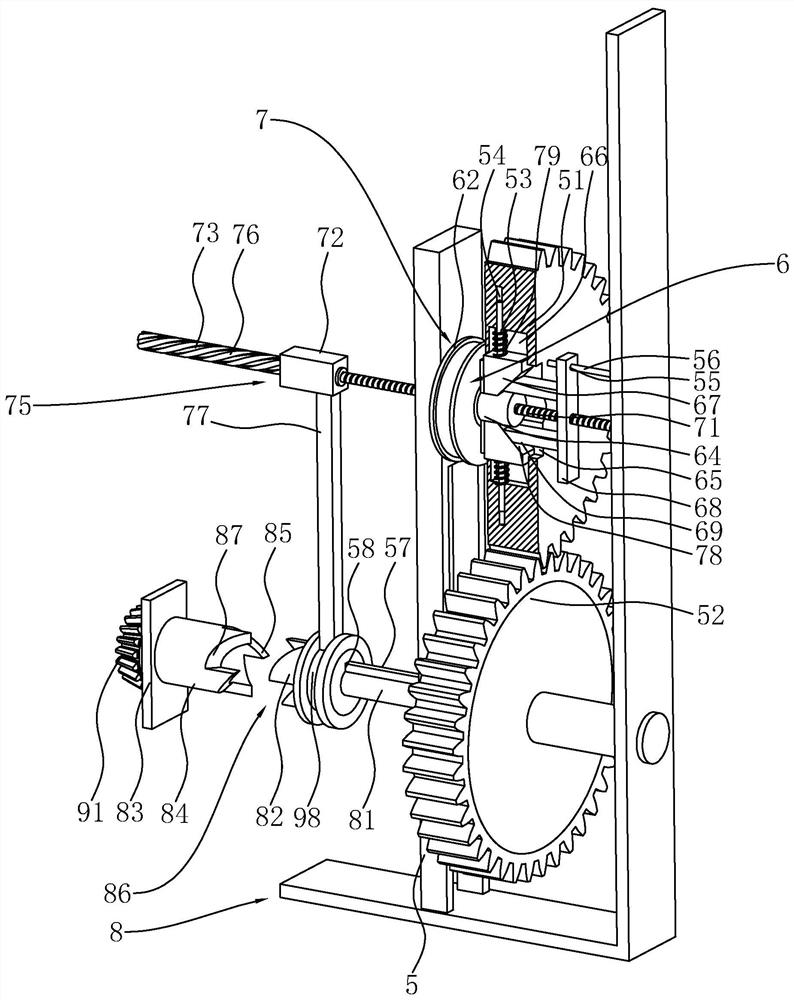
Sodium bicarbonate feeding device
ActiveCN111396911BImprove efficiencyEasy accessBulk conveyorsIncinerator apparatusImpellerGear wheel
The invention discloses a sodium bicarbonate feeding device, which relates to the technical field of waste incineration treatment. The key points of the technical scheme include a material box, a discharge barrel, a valve device, an air inlet pipe and a fan; One end of the seat is fixed with a mounting seat, the top of the mounting seat is fixed with a first motor, the output end of the first motor is fixedly connected with the fan impeller, and a lifting plate is installed in the material box to slide vertically; the valve device includes a fixed ring, a positioning column , four blade shafts and four fan-shaped blades; each blade shaft is rotatably connected with the fixed ring; a circular cavity is opened in the positioning column, and the first transmission component is arranged in the circular cavity; the top of the fixed seat is fixed with a support A seat, one side of the support seat is rotatably mounted with a first gear, one side of the mounting seat is rotatably mounted with a second gear, and the first gear meshes with the second gear. The invention solves the problem that the working efficiency of a single motor cannot be fully utilized, and achieves the effect of improving the working efficiency of the motor.
Owner:上海城投瀛洲生活垃圾处置有限公司
Biological buffering bicarbonate tablet and preparation method thereof
InactiveCN106398645ASolve the unstable outputLong term storageOther chemical processesCross-linkSodium bicarbonate
The invention relates to a biological buffering bicarbonate tablet and a preparation method thereof. The preparation method comprises the following steps: (1) accurately weighing sodium carbonate, sodium bicarbonate, cross-linked amylose and sodium azide; (2) uniformly mixing the above components under stirring; (3) pressing the components into a tablet on a tablet press; and (4) carrying out weighing and testing to obtain qualified tablets, and then carrying out packaging; wherein the pressure of the tablet press is five tons. The round tablet prepared in the invention can be directly used immediately after opening of package, is simple to use, substantially mitigates labor intensity of users, saves working time and improves working efficiency; in usage, the tablet can be rapidly dissolved after addition into a solvent with a fixed volume, so a desired buffer solution with an accurate and stable pH value can be obtained; and the tablet is easy to preserve and transport, long in preservation time, simple and rapid to prepare and suitable for industrial processing.
Owner:TIANJIN GUANGFU TECH DEV
Antibacterial environment-friendly tableware and preparation method thereof
PendingCN114395234AGood mechanical propertiesImprove antibacterial propertiesPolyvinyl alcoholEngineering
The invention discloses antibacterial environment-friendly tableware and a preparation method thereof. The preparation method comprises the following steps: weighing the following raw materials: polylactic acid, Arabic gum, sodium bicarbonate, polyvinyl alcohol, poly-3-hydroxybutyrate, calcium stearate, a coupling agent, an inorganic filler, an antibacterial agent and water; mixing and stirring the raw materials to obtain mixed slurry; injecting the mixed slurry into a mold for hot pressing, and demolding after molding; and taking out the demolded finished product, drying, trimming, deburring, and disinfecting to obtain the finished product. The antibacterial environment-friendly tableware disclosed by the invention has good mechanical property, antibacterial property and degradation property.
Owner:宁夏青林华源科技有限公司
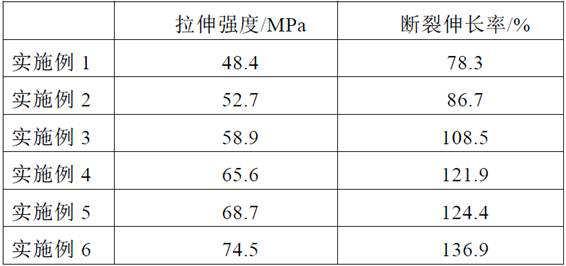
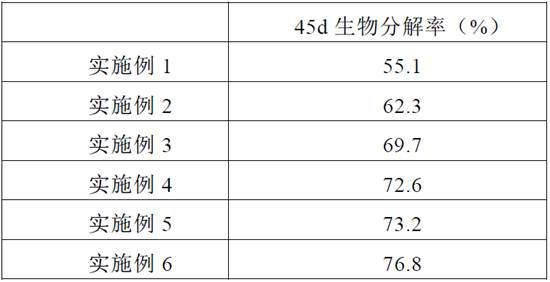
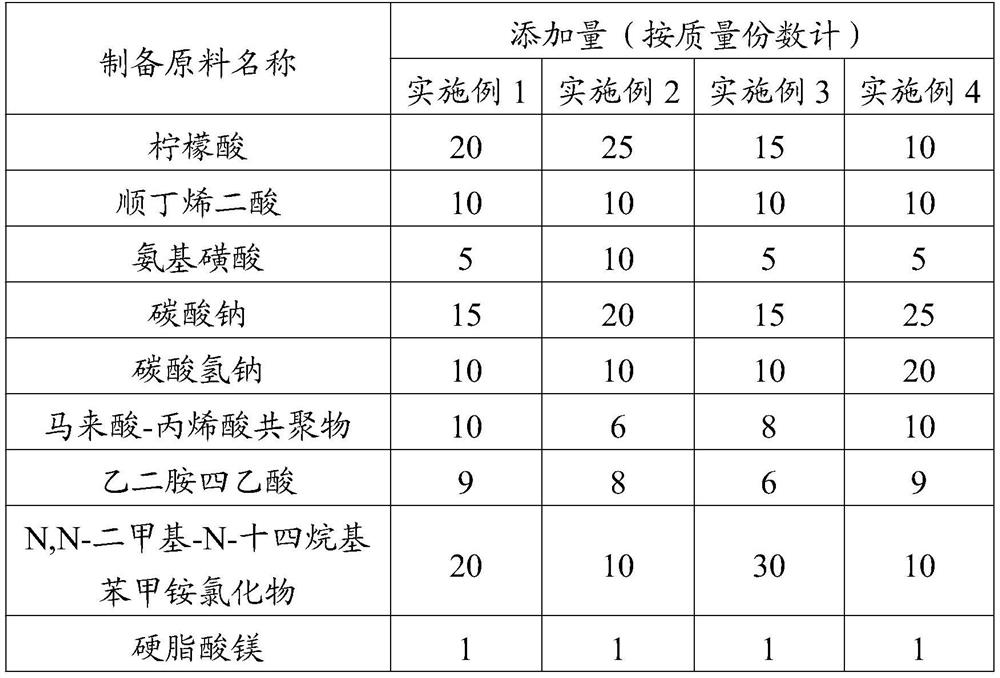
A kind of effervescent tablet and its preparation method and application
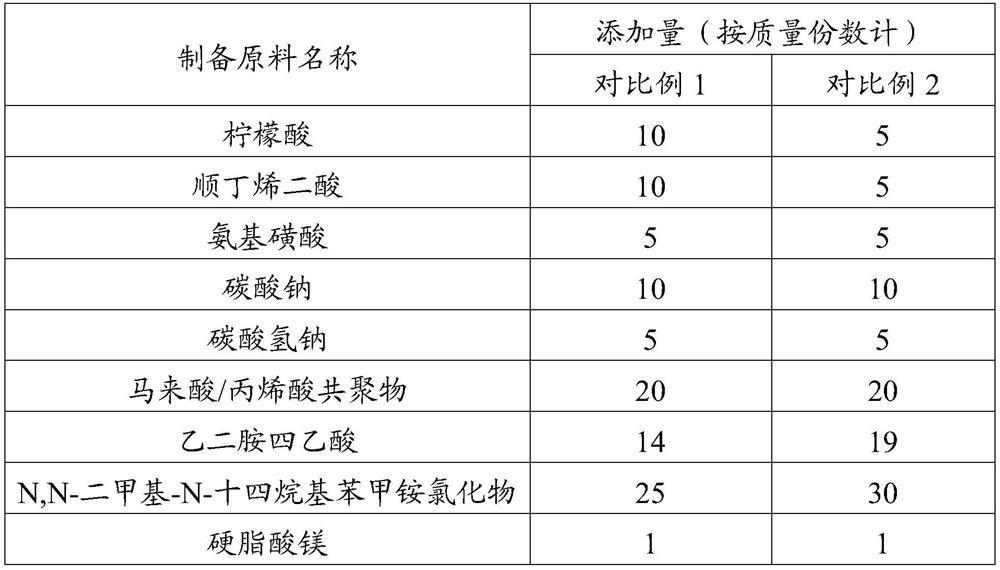
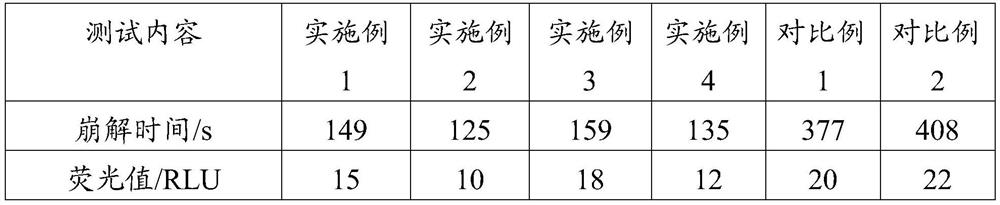
ActiveCN111286410BQuick washAvoid depositionInorganic/elemental detergent compounding agentsCationic surface-active compoundsMagnesium stearateCis-Butenedioic Acid
The invention provides an effervescent tablet and its preparation method and application, belonging to the technical field of cleaning agents. The effervescent tablet provided by the present invention comprises the following components in parts by mass: 10-30 parts of citric acid, 10-20 parts of maleic acid, 5-15 parts of sulfamic acid, and 15-25 parts of sodium carbonate , 5-20 parts of sodium bicarbonate, 1-15 parts of maleic acid-acrylic acid copolymer, 5-10 parts of ethylenediaminetetraacetic acid, 0.5-1 part of magnesium stearate, N,N-dimethyl-N- Tetradecylbenzyl ammonium chloride 5-30 parts. Utilizing the effervescent tablet provided by the present invention as a cleaning agent, it can be quickly disintegrated after being put into water, and the cleaning effect is good, especially suitable for cleaning milk dirt. When used to clean the milk circuit of a coffee machine, it can quickly and thoroughly clean milk. The remaining milk dirt in the road prevents dirt from depositing and prolongs the service life of the coffee machine.
Owner:南京卫岗乳业有限公司
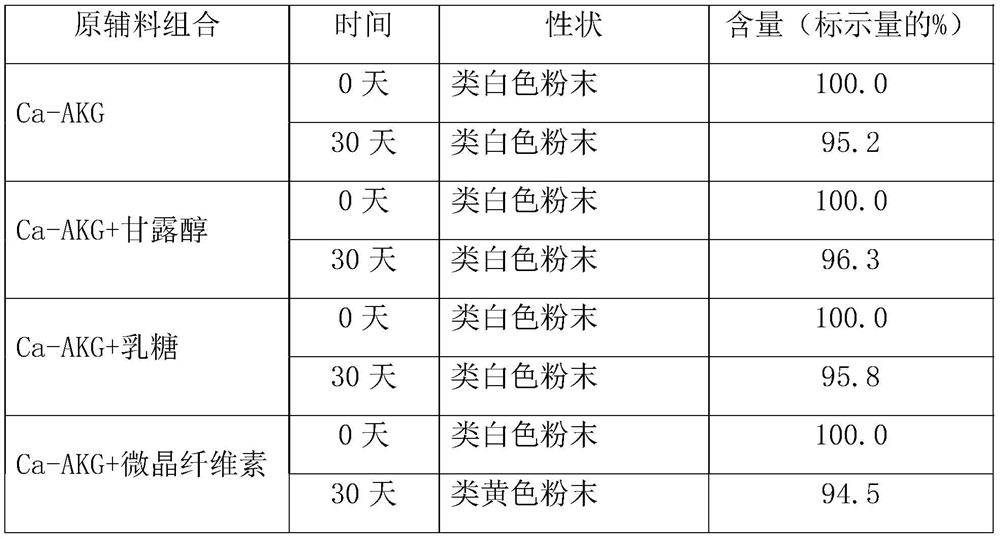
Ca-AKG effervescent tablet and preparation method thereof
The invention relates to a Ca-AKG effervescent tablet. TheCa-AKG effervescent tablet is prepared by taking alpha-ketoglutarate as a raw material medicine and sodium bicarbonate, mannitol, citric acid, orange essence, aspartame, silicon dioxide, polyethylene glycol 6000, sunset yellow pigment and an adhesive as auxiliary materials. The invention further relates to a preparation method of the Ca-AKG effervescent tablet. The preparation method comprises the steps of wet granulation, drying, size stabilization, total mixing, tabletting, packaging and the like. The Ca-AKG effervescent tablet is stable in property and suitable for clinical application, the preparation method is easy and convenient to operate and good in reproducibility, and the prepared product is uniform in quality.
Owner:ABA CHEM CORP +1
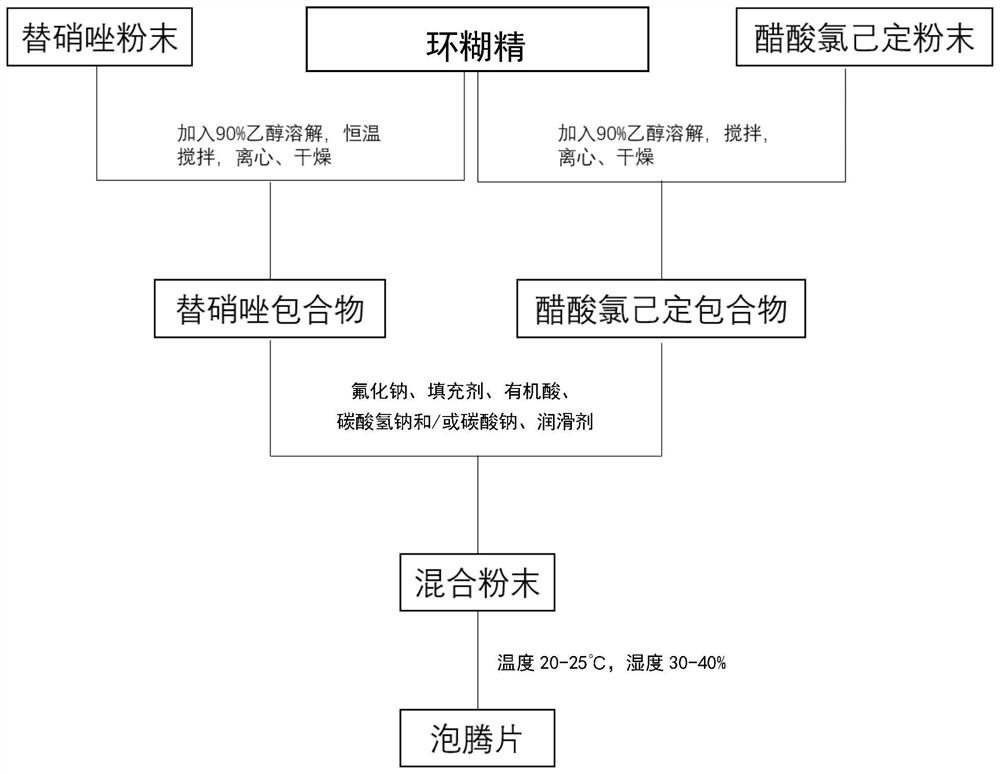
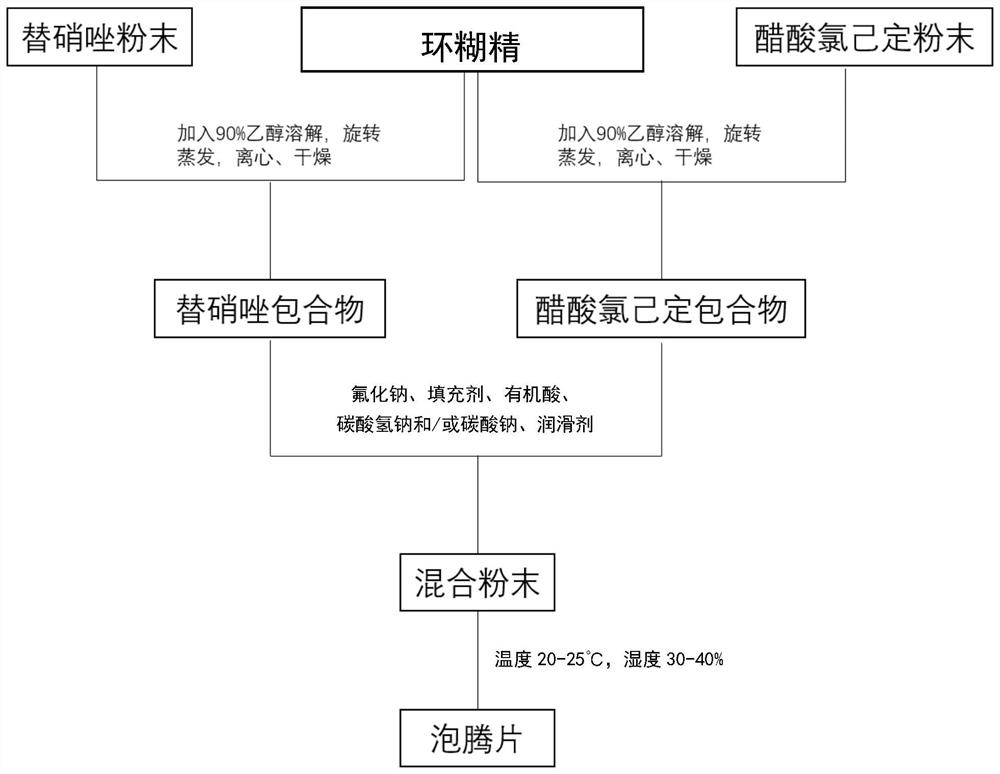
Composite effervescent tablet as well as preparation method and application thereof in gargling
PendingCN114788833AEasy to useEasy to carryAntibacterial agentsCosmetic preparationsChlorhexidine AcetateCyclodextrin
The invention discloses a composite effervescent tablet which comprises tinidazole, chlorhexidine acetate, sodium fluoride, cyclodextrin, a filler, organic acid, sodium bicarbonate and / or sodium carbonate and a lubricant. The invention further discloses a preparation method of the compound effervescent tablet and application of the compound effervescent tablet in gargling. The composite effervescent tablet containing tinidazole and chlorhexidine acetate is high in dissolution speed, high in solution clarity, convenient to carry and long-acting in quality guarantee, not only has the effects of cleaning, diminishing inflammation and resisting bacteria, but also has the effects of preventing decayed teeth and strengthening teeth, and is wide in application prospect.
Owner:SHANGHAI CHANGZHENG HOSPITAL +1
A kind of edaravone sodium chloride injection and preparation method thereof
ActiveCN110090225BImprove neurological symptomsImprove medication safetyOrganic active ingredientsNervous disorderVitamin CSodium Chloride Injection
The invention relates to an edaravone sodium chloride injection and a preparation method thereof. 100ml of the injection includes: edaravone 20-40mg, sodium chloride 0.6-1.0g, propylene glycol 100-150mg, vitamin C 2.5-3.5g, sodium bicarbonate 0.3-1.0g, pH adjusted to 3.5-5.0. The preparation process of the injection comprises: dosing, filtering, filling, capping, sterilizing, and lamp inspection; the dosing adopts a two-step preparation method, and the preparation process adopts low-temperature preparation. The method is simple to operate and low in cost, and the prepared Edaravone Sodium Chloride Injection has good drug stability, less side effects, effectively reduces the production of impurities, and has high drug compliance and safety for patients. It is used to improve acute cerebral palsy Neurological symptoms, activities of daily living and dysfunction caused by infarction can effectively prevent and treat brain injury.
Owner:JINAN KANGHE MEDICAL TECH
Pharmaceutical preparation of cefotaxime sodium compound and preparation method thereof
PendingCN114209651AImprove liquidityEncapsulation rate unchangedAntibacterial agentsOrganic active ingredientsCEFMINOX SODIUMCholesterol
The invention discloses a pharmaceutical preparation of a cefotaxime sodium compound. The pharmaceutical preparation comprises the following raw materials: 10 parts of cefotaxime sodium; 57.86 parts of N-(beta-D-glucopyranose) octanamide, and 57.86 parts of N-(beta 19.29 parts of octadecylamine; 162.14 parts of 1-palmitoyl-2-stearoyl lecithin, and 162.14 parts of 1-palmitoyl-2 27.14 parts of mannitol; 80.71 parts of cholesterol; 62.86 parts of tartaric acid; and 15.71 parts of solid sodium bicarbonate particles. In addition, the invention also discloses a preparation method of the medicinal preparation. The cefminox sodium pharmaceutical preparation is better in flowability; meanwhile, after long-time storage (for example, more than three months), the drug encapsulation efficiency is basically kept unchanged.
Owner:上海欣峰制药有限公司
A kind of preparation method of o-iodoaniline
ActiveCN106542958BWide variety of sourcesLow priceHalogenated hydrocarbon preparationPtru catalystBiochemical engineering
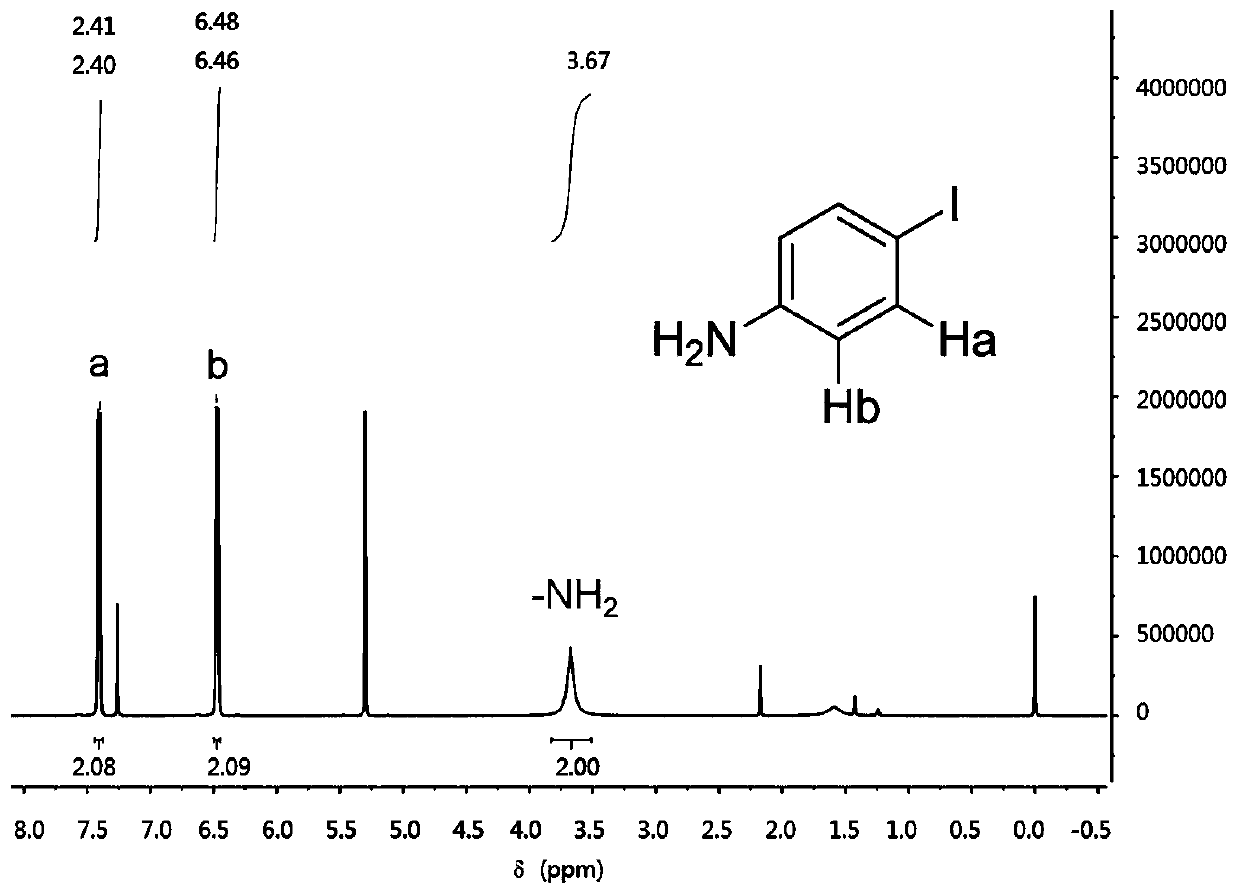
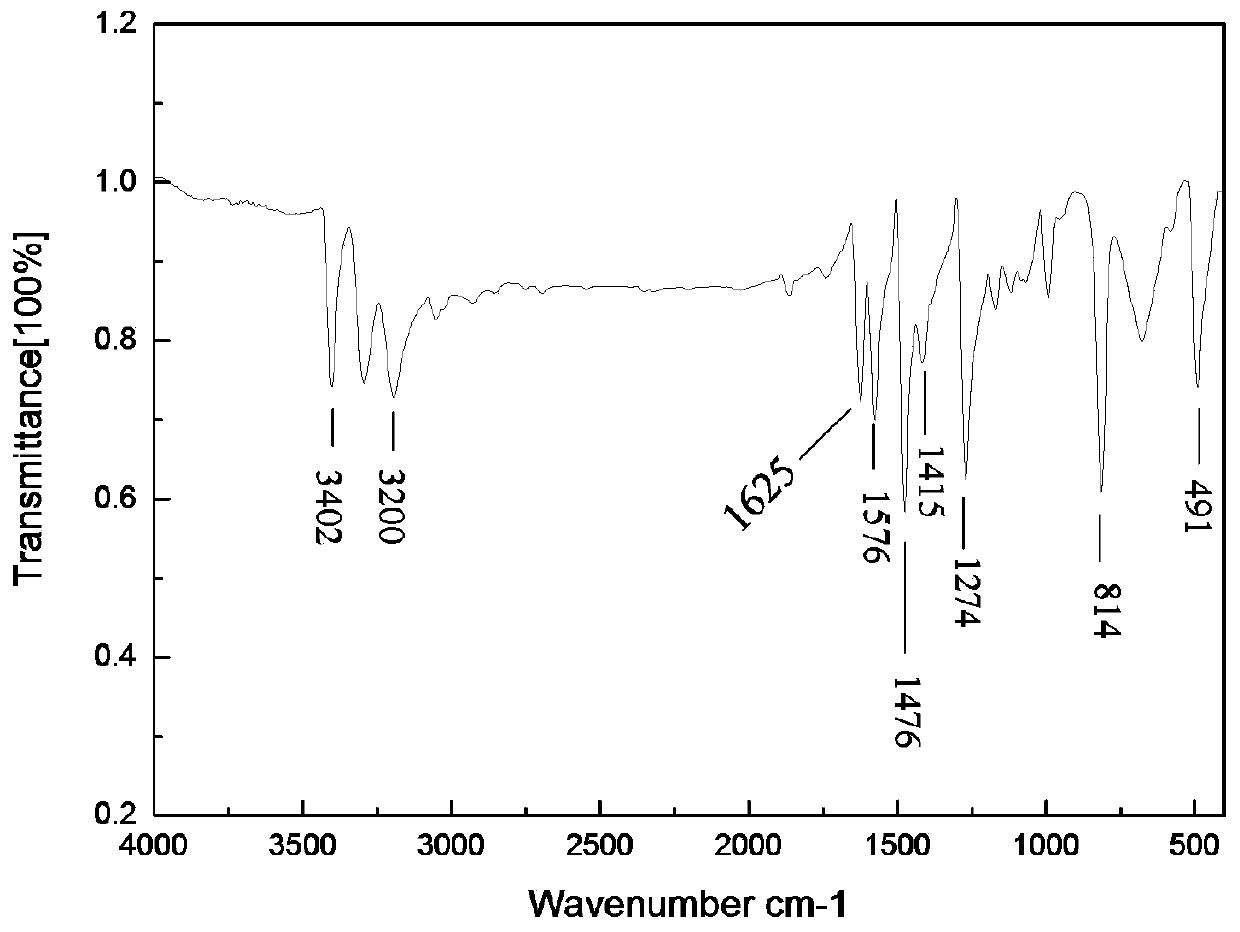
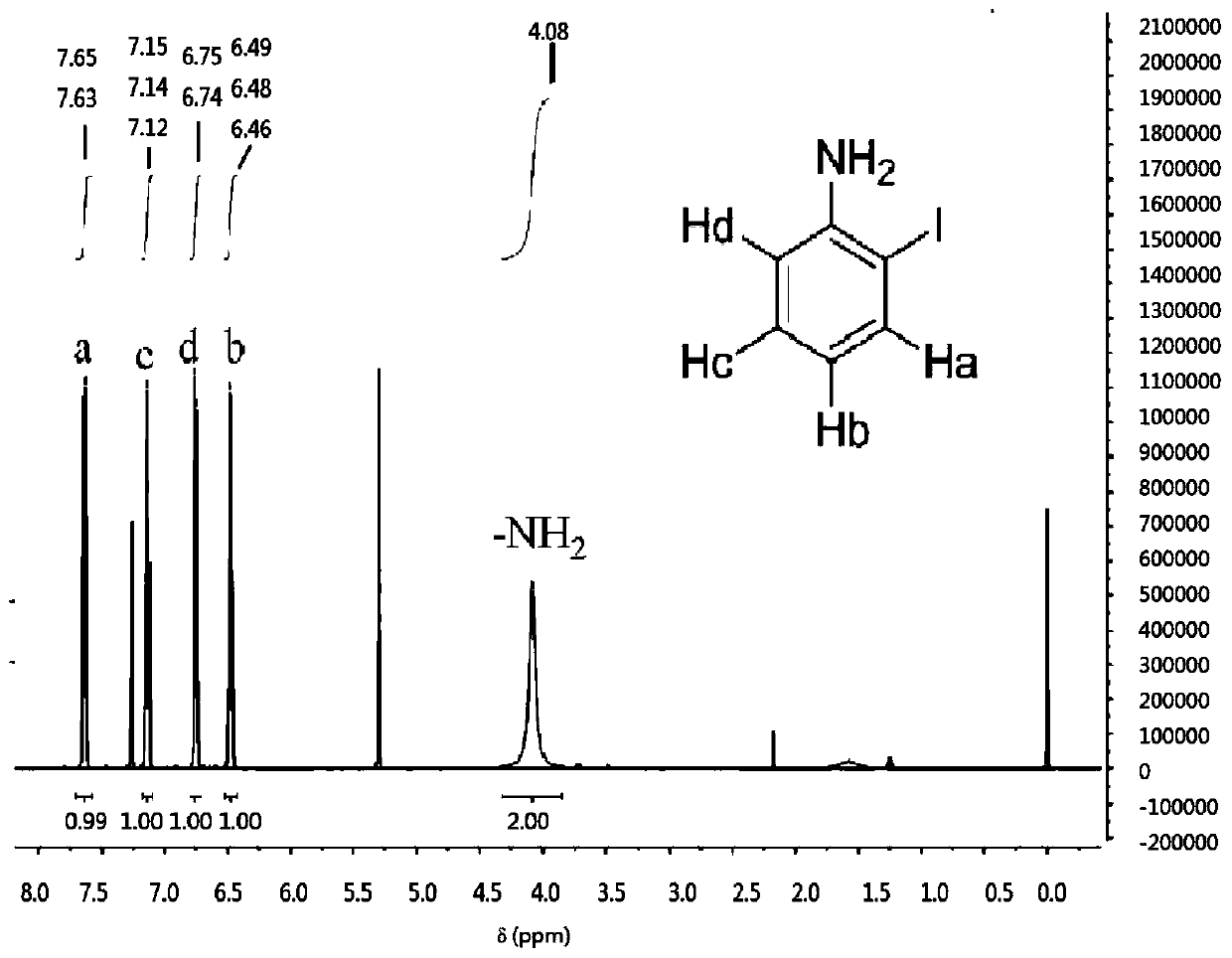
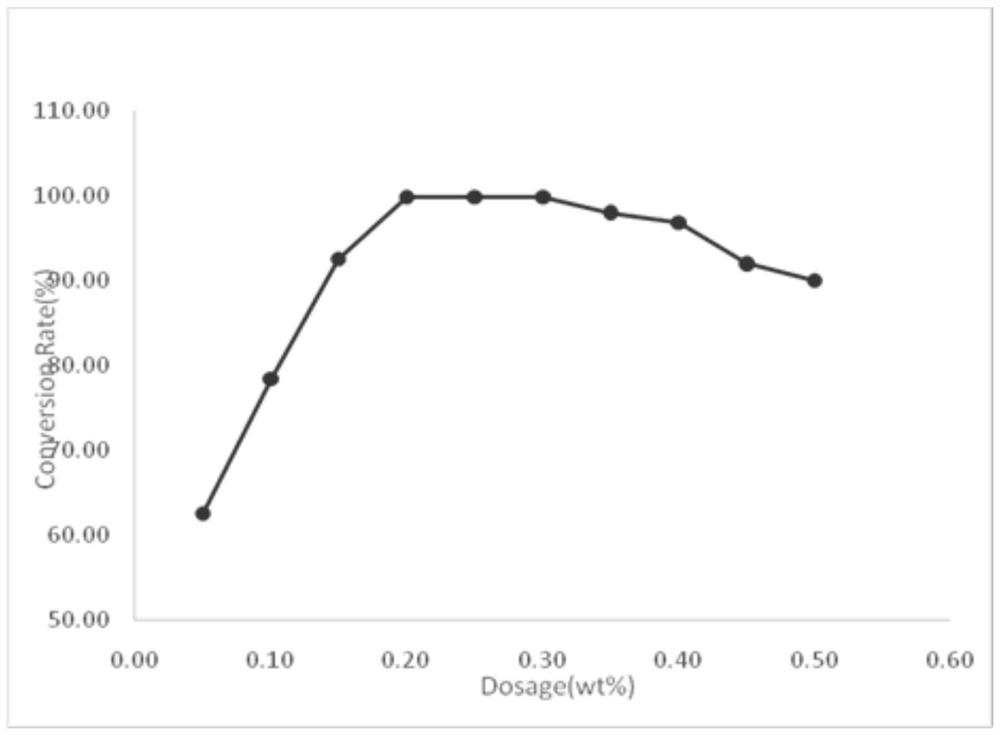
The invention discloses a preparation method of o-iodoaniline. The synthesis route is represented by a formula in the invention. The preparation method possesses following advantages: raw material aniline is widely available and cheap, and is beneficial for industrialized utilization of the preparation method; in a certain solvent, sodium bicarbonate / I2 are used for preparing paraiodoaniline via direct iodination of aniline, and yield is high; paraiodoaniline is taken as a raw material, a certain catalyst is adopted, and rearrangement reaction is carried out so as to realize chemical conversion of paraiodoaniline into o-iodoaniline, processing steps are few, conditions are mild, and green environment-protection requirements are satisfied; and both paraiodoaniline and o-iodoaniline are preferable pharmaceutical intermediates.
Owner:GUIZHOU UNIV
Polystyrene seed emulsion and preparation method thereof
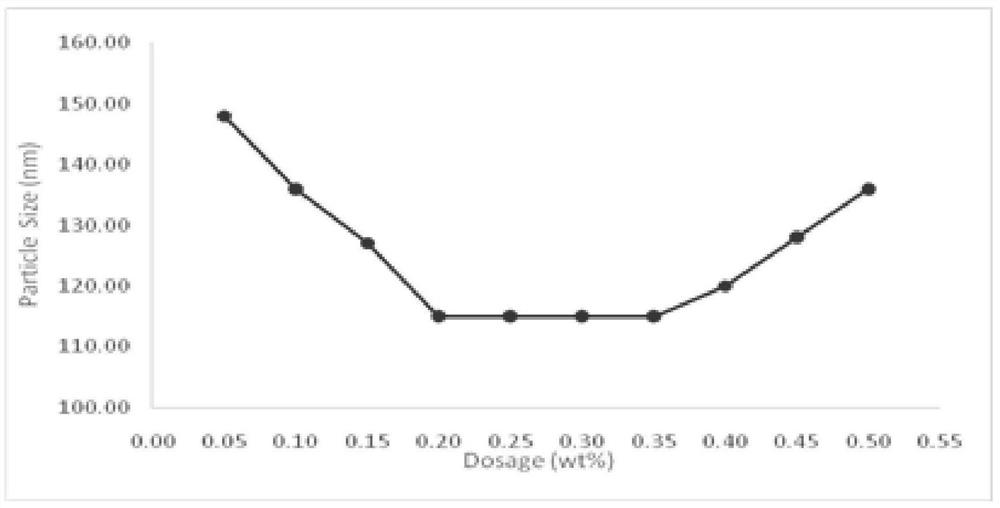
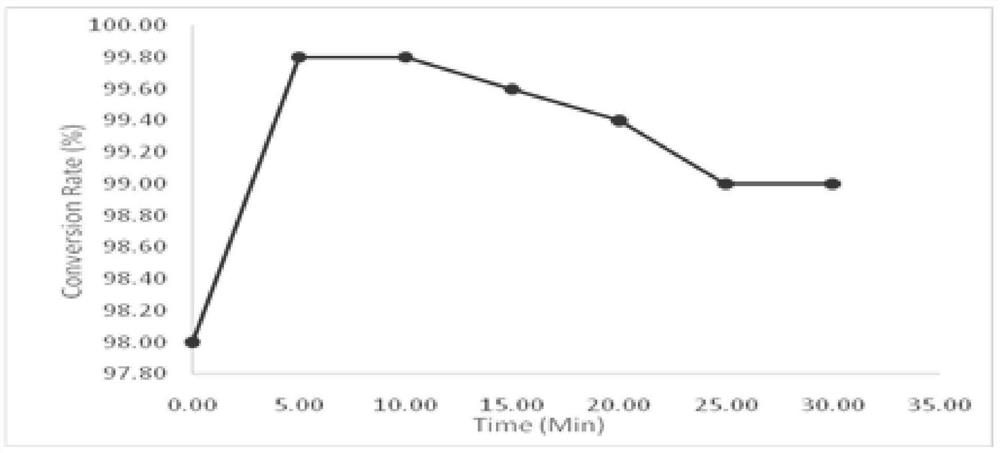
The invention relates to a polystyrene seed emulsion, which is prepared from the following raw materials in parts by weight: 500-600 parts of a styrene monomer, 4-10 parts of t-DDM, 0.5-1 part of sodium bicarbonate, 200-220 parts of Disponil SLS103, 1-2 parts of potassium persulfate and 1200-1300 parts of water. The invention also provides a preparation method of the polystyrene seed emulsion. The operation steps are simple, the raw materials are easy to obtain, the cost is low, and the polystyrene seed emulsion with high monomer conversion rate and small particle size can be obtained through the method. It is found that the time for adding an initiator into an emulsion system plays an important role in monomer conversion rate and particle size distribution.
Owner:广东天银实业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com