Composite effervescent tablet as well as preparation method and application thereof in gargling
A technology of effervescent tablets and clathrates, which is applied in the fields of drug combination, oral cavity care, and pharmaceutical formulations. It can solve the problems of fast dissolution, high solution clarity, and inconvenient portability of mouthwash, etc., so that it is not easy to carry and improves solubility. , easy to carry effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
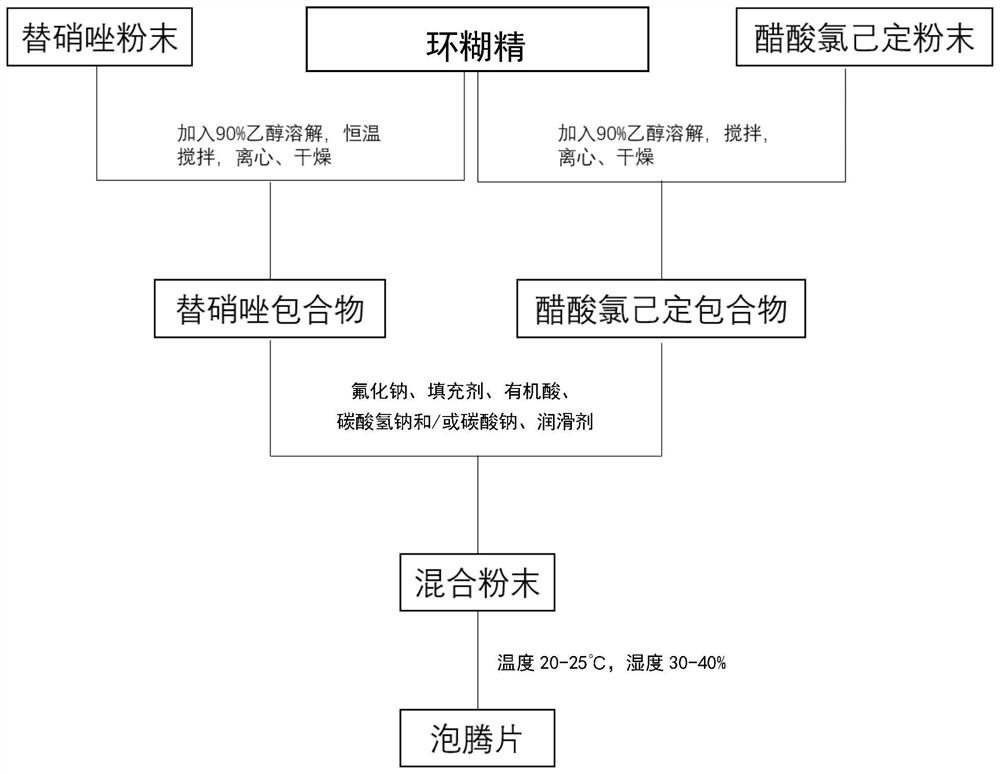
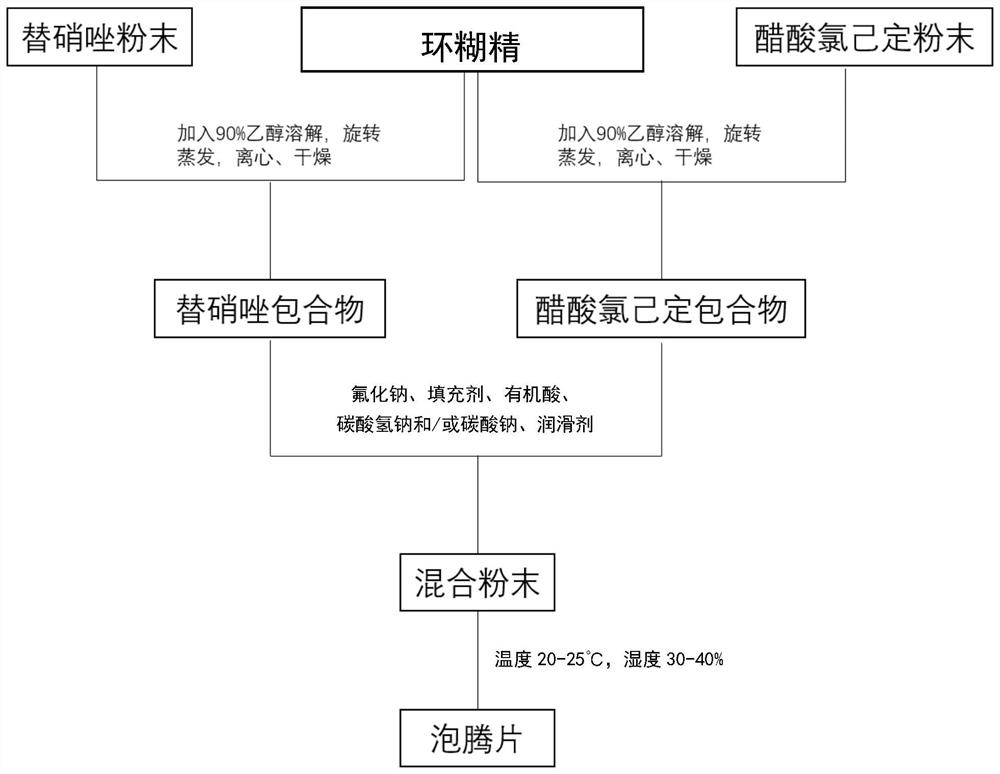
[0058] like figure 1 As shown, in one embodiment of the present invention, the preparation method of the composite effervescent tablet containing tinidazole and chlorhexidine acetate, comprises the following steps:
[0059] Weigh tinidazole or chlorhexidine acetate: hydroxypropyl-β-cyclodextrin in a molar ratio of 1:1-1:2, put it into a round-bottomed flask, add 10-20ml of 90% ethanol solution, and place it at 40 Stir at a constant temperature of 30-80rpm for 2-4h in a -60°C water bath, cool, remove unincorporated tinidazole or chlorhexidine acetate by high-speed centrifugation, evaporate the solvent, and place it in a vacuum drying box to dry for 8h-16h. Nitazole or chlorhexidine acetate inclusion complex powder, ground with a mortar and sieved;
[0060] Weigh sodium fluoride, filler, organic acid, sodium bicarbonate and / or sodium carbonate, lubricant in proportion, add tinidazole clathrate and chlorhexidine acetate clathrate, and mix to obtain mixed powder;
[0061] Under ...
Embodiment 1
[0067] The raw materials and their percentages (%) are as follows (the raw materials used are all medicinal specifications, and the reagents are of analytical grade):
[0068] Tinidazole 1.25, chlorhexidine acetate 1.25, hydroxypropyl-β-cyclodextrin 27.00, sodium fluoride 2.50, tartaric acid 29.00, sodium bicarbonate 33.00, PEG6000 1.00, lactose 5.00.
[0069] Weigh tinidazole and chlorhexidine acetate respectively according to the percentage content, add hydroxypropyl-β-cyclodextrin respectively, carry out inclusion operation by constant temperature stirring method, then dry, grind and sieve, then add sodium fluoride, Tartaric acid, sodium bicarbonate, lactose, and PEG6000 are mixed, the indoor temperature is lowered to below 20° C. and the humidity is lowered to below 35%, and direct tableting is performed.
[0070] The tableting process was smooth without sticking punching, splitting and other phenomena. The disintegration time of the prepared tablet was 1 minute and 42 sec...
Embodiment 2
[0072] The raw materials and their percentages (%) are as follows (the raw materials used are all medicinal specifications, and the reagents are of analytical grade):
[0073] Tinidazole 2.5, chlorhexidine acetate 2.5, methyl-β-cyclodextrin 13.00, sodium fluoride 5.00, citric acid 39.00, sodium carbonate 34.00, sodium lauryl sulfate 2.00, cellulose 2.00.
[0074] Weigh tinidazole and chlorhexidine acetate respectively according to the percentage content, add methyl-β-cyclodextrin and carry out inclusion operation by rotary evaporator, then dry, grind and sieve, then add sodium fluoride, lemon Acid, sodium carbonate, cellulose, and sodium lauryl sulfate are mixed, the indoor temperature is lowered to below 20° C. and the humidity is lowered to below 35%, and direct tableting is performed.
[0075] The tableting process was smooth without sticking punching, splitting and other phenomena. The disintegration time of the prepared tablet was 1 minute and 48 seconds, and the solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com