A composition containing linezolid and its preparation method
A technology of linezolid and its composition, which is applied in the field of linezolid tablets and its preparation, can solve the problems of poor compressibility, easy change of linezolid crystal form, slow disintegration speed, etc., and achieves low cost and is suitable for industrial production , the effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
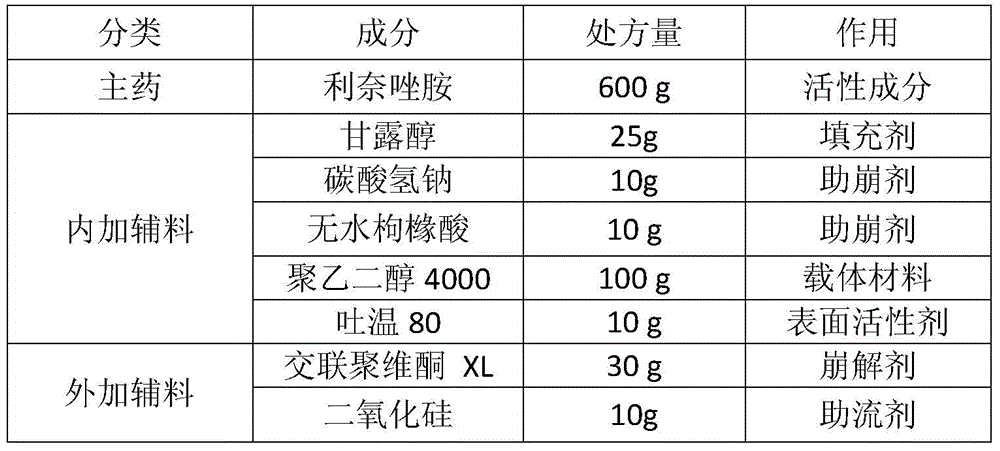
[0030] Prescription (1000 tablets)
[0031]
[0032] Process:
[0033] The linezolid raw material is micronized together with mannitol, sodium bicarbonate and anhydrous citric acid to a suitable particle size (D90 is less than 30 μm).
[0034] First heat polyethylene glycol 4000 to melt (65°C±5°C), add Tween 80 and stir evenly, keep warm. Then put the above micronized mixture into a fluidized bed for fluidization, the air inlet temperature is 45-55°C, and at the same time spray the above molten liquid at a speed of 8-12g / min to prepare solvent-free spray particles. Then add crospovidone XL and silicon dioxide, mix evenly and press into tablets.
example 2
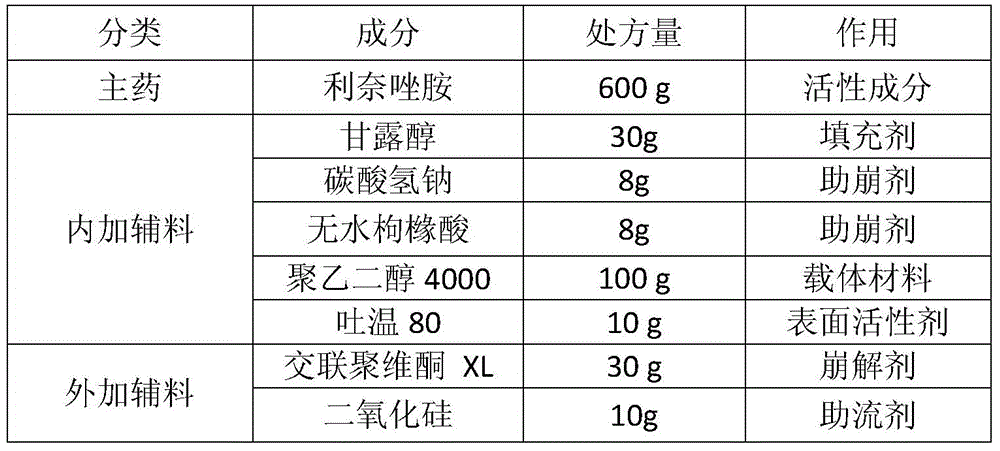
[0036] Prescription (1000 tablets)
[0037]
[0038] Process:
[0039] The linezolid raw material is micronized together with mannitol, sodium bicarbonate and anhydrous citric acid to a suitable particle size (D90 is less than 40 μm).
[0040] First heat polyethylene glycol 4000 to melt (65°C±5°C), add Tween 80 and stir evenly, keep warm. Then put the above micronized mixture into a fluidized bed for fluidization, the air inlet temperature is 50-60°C, and at the same time spray the above molten liquid at a speed of 5-8g / min to prepare solvent-free spray particles. Then add crospovidone XL and silicon dioxide, mix evenly and press into tablets.
example 3
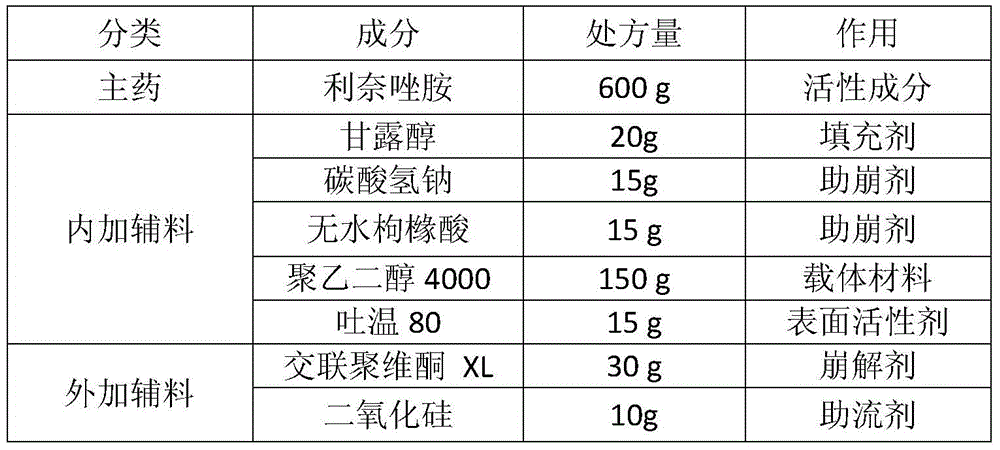
[0042] Prescription (1000 tablets)
[0043]
[0044] Process:
[0045] The linezolid raw material is micronized together with mannitol, sodium bicarbonate and anhydrous citric acid to a suitable particle size (D90 is less than 30 μm).
[0046] First heat polyethylene glycol 4000 to melt (65°C±5°C), add Tween 80 and stir evenly, keep warm. Then put the above micronized mixture into a fluidized bed for fluidization, the air inlet temperature is 45-55°C, and at the same time spray the above molten liquid at a speed of 11-14g / min to prepare solvent-free spray particles. Then add crospovidone XL and silicon dioxide, mix evenly and press into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com