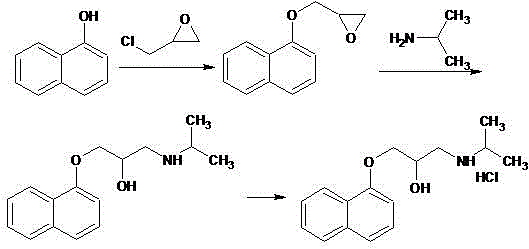
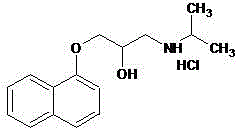
Novel propranolol synthesis method
A new method and naphthyloxy technology, applied in the field of synthesizing propranolol, can solve the problems of low recovery rate, poor recovery effect, easy loss, etc., and achieve the effects of less side reactions, less pollution, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh 200.2 g of 3-(1-naphthyloxy)-1,2-propylene oxide, 70.9 g of isopropylamine and 1800 ml of dichloromethane, and place them in a reaction flask. With the temperature controlled below 30°C, 4.0 g of triethylamine was slowly added dropwise with stirring. After dropping, keep warm at 25-30°C and stir for reaction. The reaction was monitored by TLC. After 5 hours, the reaction was stopped. The residue was evaporated to dryness under reduced pressure, and the residue was recrystallized from toluene / n-hexane to obtain 243.3 g of propranolol, with a yield of 93.8% and an HPLC purity of 99.3%.
Embodiment 2
[0027] Weigh 200.2 g of 3-(1-naphthyloxy)-1,2-propylene oxide, 76.8 g of isopropylamine and 1000 ml of dichloromethane, and place them in a reaction flask. With the temperature controlled below 30°C, 2.3 g of triethylamine was slowly added dropwise with stirring. After dropping, keep warm at 25-30°C and stir for reaction. The reaction was monitored by TLC. After 5.5 hours, the reaction was stopped. The residue was evaporated to dryness under reduced pressure, and the residue was recrystallized from toluene / n-hexane to obtain 244.0 g of propranolol, with a yield of 94.1% and an HPLC purity of 99.2%.
Embodiment 3
[0029] Weigh 200.2 g of 3-(1-naphthyloxy)-1,2-propylene oxide, 59.1 g of isopropylamine and 1600 ml of dichloromethane, and place them in a reaction flask. With the temperature controlled below 30°C, 2.5 g of triethylamine was slowly added dropwise with stirring. After dropping, keep warm at 25-30°C and stir for reaction. The reaction was monitored by TLC. After 6 hours, the reaction was stopped. The residue was evaporated to dryness under reduced pressure, and the residue was recrystallized from toluene / n-hexane to obtain 241.2 g of propranolol, with a yield of 93.0% and an HPLC purity of 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com