Pharmaceutical composition for resisting methicillin-resistant staphylococcus aureus (mrsa)
A technology of anti-methicillin and composition, applied in the directions of drug combination, antibacterial drug, active ingredient of heterocyclic compounds, etc., can solve the problem that the treatment cannot meet the clinical needs, etc., and achieve the effect of preventing and treating infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
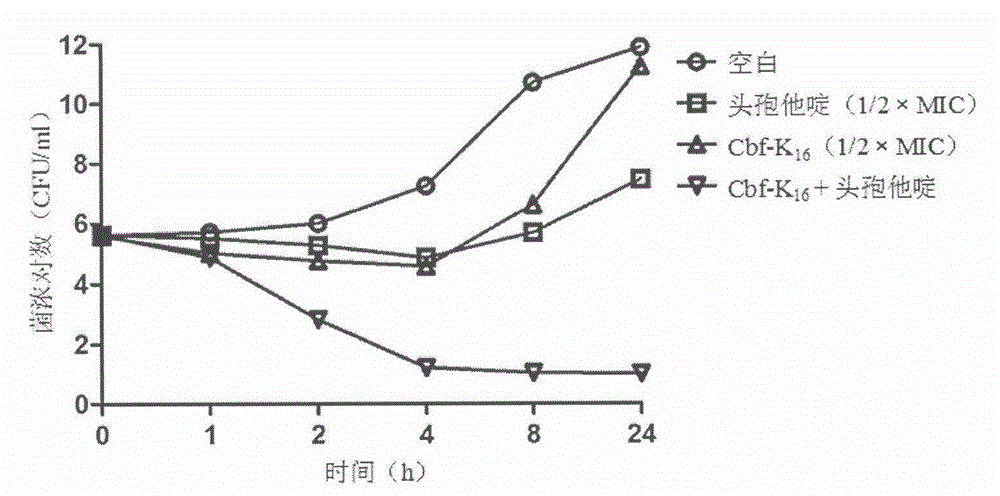
[0047] Cbf-K 16 Effect of combined use with ceftazidime on the death protection rate of MRSA-infected mice
[0048] The experiment uses about 20g of ICR mice, half male and half male, randomly divided into 7 groups, set as blank group, model group, Cbf-K 16 group (40mg / kg / d), ceftazidime group (80mg / kg / d), low dose combination group (Cbf-K 16 20mg / kg / d, ceftazidime 80mg / kg / d), medium-dose combination group (Cbf-K 16 40mg / kg / d, ceftazidime 80mg / kg / d), high-dose combination group (Cbf-K 16 80mg / kg / d, ceftazidime 80mg / kg / d). After 2 days of adaptive culture, the experiment was started. Except for the blank group, mice in other groups were intraperitoneally injected with 5×10 8 CFU of MRSA, Cbf-K 16 0.5h and 2h after infection were administered intraperitoneally, ceftazidime was administered via tail vein at 0h and 6h after infection, the survival status was observed for 7 consecutive days, the number of deaths was recorded, and the death protection rate was calculated.
[...
Embodiment 2
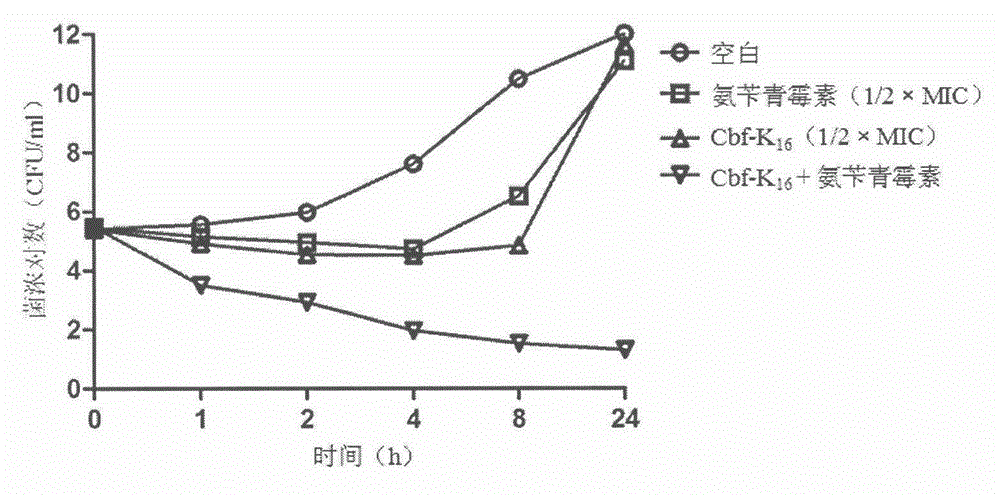
[0053] Cbf-K 16 Effects of combined use with ampicillin on the death protection rate of MRSA-infected mice
[0054] The experiment uses about 20g of ICR mice, half male and half male, randomly divided into 7 groups, set as blank group, model group, Cbf-K 16 group (40mg / kg / d), ampicillin group (80mg / kg / d), low-dose combination group (Cbf-K 16 20mg / kg / d, ampicillin 80mg / kg / d), medium-dose combination group (Cbf-K 16 40mg / kg / d, ampicillin 80mg / kg / d), high-dose combination group (Cbf-K 16 80mg / kg / d, ampicillin 80mg / kg / d). After 2 days of adaptive culture, the experiment was started. Except for the blank group, mice in other groups were intraperitoneally injected with 5×10 8 CFU of MRSA, Cbf-K 16 0.5h and 2h after infection, intraperitoneal administration, ampicillin 0h and 6h after infection tail vein administration, 7 days to observe the survival status, record the number of deaths, and calculate the death protection rate.
[0055] Table 5 Cbf-K 16 and ampicillin on the d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com