High sulfur-resistant catalyst for using syngas to produce methane, preparation method and application
A catalyst and methane production technology, applied in the direction of hydrocarbon production from carbon oxides, etc., can solve the problems of poor activity, inability to directly apply coal-to-natural gas methanation process, low sulfur tolerance, etc., achieve strong sulfur tolerance and simple preparation Ease of operation and reduction of preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
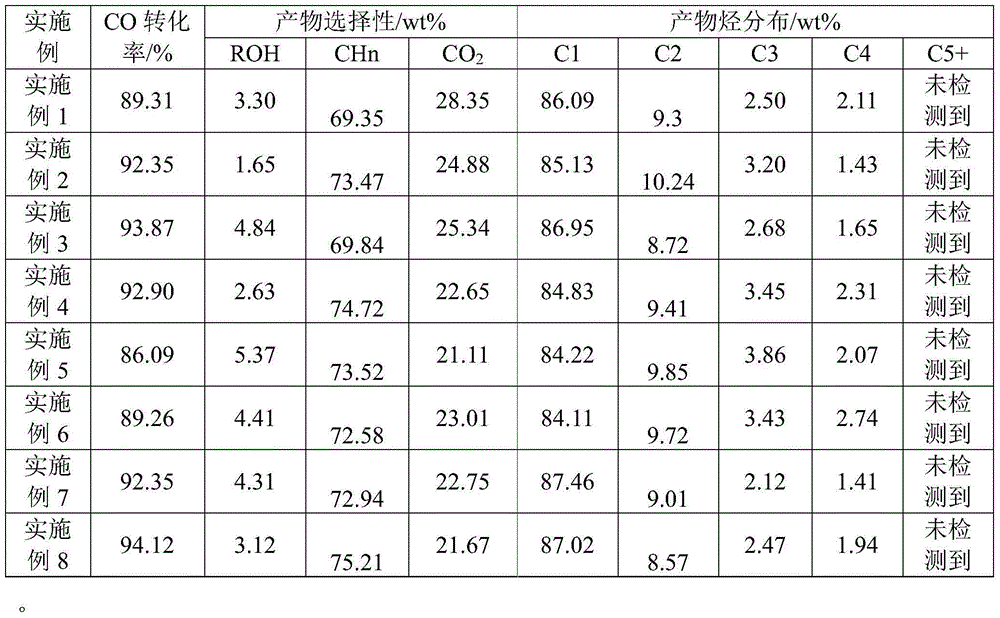
Embodiment 1
[0036] At room temperature, mix 100g of pseudo-boehmite with 60ml of dilute nitric acid with a concentration of 6%, knead and extrude into strips, dry naturally at room temperature for 24 hours, then dry at 120°C for 12 hours, and then bake at 450°C 10 hours, produced Al 2 o 3 carrier.
[0037] The obtained carrier is pulverized and ground to 40-60 mesh. According to MoO in the final sample 3 The mass percentage is 10%, V 2 o 5 The mass percentage is calculated as 5%, and the corresponding amount of (NH 4 ) 6 Mo 7 o 24 4H 2 O and NH 4 VO 3 reagent. The (NH 4 ) 6 Mo 7 o 24 4H 2 The O solution was impregnated on the above-mentioned carrier, impregnated at room temperature for 6 hours, then dried at 60° C. for 6 hours, and then dried at 120° C. for 12 hours. After drying, it was baked at 500° C. for 4 hours in an air atmosphere. Then the auxiliary component NH4 VO 3 The solution was impregnated with the sample obtained above by equal volume method, impregnated...
Embodiment 2
[0040] At room temperature, mix 100g of pseudo-boehmite with 70ml of 5% dilute nitric acid, knead and extrude into strips, dry naturally at room temperature for 16 hours, then dry at 80°C for 12 hours, and then bake at 550°C 6 hours, produced Al 2 o 3 carrier.
[0041] The obtained carrier is pulverized and ground to 40-60 mesh. According to MoO in the final sample 3 The mass percentage composition is 25%, and the NiO mass percentage composition is 0.3% to calculate, take corresponding amount (NH 4 ) 6 Mo 7 o 24 4H 2 O and NH 4 VO 3 reagent. Simultaneously (NH 4 ) 6 Mo 7 o 24 4H 2 O and Ni(NO 3 ) 2 ·6H 2 O solution was impregnated in the prepared Al 2 o 3 On the carrier, it was immersed at room temperature for 4 hours, then dried at 50°C for 10 hours, and then dried at 120°C for 8 hours. After drying, it was baked at 400° C. for 8 hours in an air atmosphere. Finally, the catalyst composition is MoO 3 :NiO:Al 2 o 3 =25:0.3:74.7 (mass ratio), recorded as...
Embodiment 3
[0044] Co-precipitation products were prepared by using 1.75 mol / L zirconium nitrate solution and 0.75 mol / L ammonia water at 40°C with continuous stirring. The obtained precipitate was aged for 4 hours, then suction filtered, washed, and then calcined at 500°C for 4 hours in the air to obtain ZrO 2 carrier.
[0045] Will produce ZrO 2 The carrier is pulverized and ground to 40-60 mesh. According to MoO in the final sample 3 The mass percentage is 1%, Co 2 o 3 The mass percentage composition is calculated as 24%, and the corresponding amount (NH 4 ) 6 Mo 7 o 24 4H 2 O and Co(NO 3 ) 2 ·6H 2 O reagent. First, an appropriate concentration of (NH 4 ) 6 Mo 7 o 24 4H 2 O impregnated in the above-prepared ZrO 2 The carrier was soaked at room temperature for 8 hours, dried at 80°C for 4 hours, and then dried at 120°C for 14 hours. The dried catalyst was calcined at 600°C for 9 hours in an air atmosphere. Then Co(NO 3 ) 2 ·6H 2 O was also impregnated with the sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com