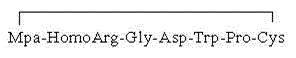Novel reduction-oxidation system for preparing Eptifibatide
A technology of eptifibatide and oxidation system, which is applied in the field of new reduction-oxidation system for the preparation of eptifibatide, can solve the problems of high difficulty in crude peptide purification and low yield, and achieve easy large-scale production and reduce production costs , the effect of reducing the difficulty of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of Fmoc-Cys(Trt)-Rink Amide AM resin
[0035] Accurately weigh 600 g of Rink Amide AM resin (initial substitution degree 0.97 mmol / g) and place it in a peptide resin synthesis reactor, add 6LDCM to swell for 2 h. After the swelling is completed, wash with DMF three times, 5 L each time, and then add 5 L of 20% piperidine / DMF solution for deprotection twice, for 10 min and 10 min respectively. After the deprotection was completed, the resin was washed 6 times with DMF, 5 L each time. Weigh 852g of Fmoc-Cys(Trt)-OH and 196g of HOBt and dissolve it in 4LDMF, add 250mL of DIC to activate, add the solution into the reactor, react for 2h, and monitor the reaction progress by Kaiser test. After the reaction was completed, the resin was washed with DMF for 3 times, and then the pre-prepared capping reagent (1 L of acetic anhydride, 850 mL of pyridine, 3 L of DMF) was added, and the capping reaction was carried out for 40 minutes. After the capp...
Embodiment 2
[0036] Example 2: Preparation of linear eptifibatide resin with fully protected side chains
[0037]Weigh 429g (300mmol) of the Fmoc-Cys(Trt)-Rink Amide AM resin with a substitution degree of 0.7mmol / g in Example 1 and place it in a peptide resin synthesis reactor, add 4LDCM to swell for 2h. After swelling, wash 3 times with DMF, 4L each time, and then add 4L of 20% piperidine / DMF solution to deprotect twice, for 5min and 5min respectively.
[0038] The abbreviations used in the specification and claims have the following meanings:
[0039] Fmoc 9-fluorenylmethoxycarbonyl
[0040] OtBu tert-butoxy Boc tert-butoxycarbonyl
[0041] Pbf 2,2,4,6,7-pentamethylbenzofuran-5-sulfonyl
[0042] Trt Trityl
[0043] DIC N,N-Diisopropylcarbodiimide
[0044] HOBt 1-Hydroxybenzotriazole
[0045] DTT dithiothreitol
[0046] DMSO dimethyl sulfoxide
[0047] Embodiment 1: the preparation of Fmoc-Cys(Trt)-Rink Amide AM resin
[0048] Accurately weigh 600 g of Rink Amide AM resin (in...
Embodiment 5
[0051] Embodiment 5: the preparation of eptifibatide fine peptide
[0052] After the oxidation reaction solution in Example 4 was filtered with a 0.45 μm microporous membrane, a C18 preparation column with an internal diameter of 100 mm was selected, purified, salted, and freeze-dried to obtain 18.5 g of eptifibatide fine peptide, with a purity greater than 99.8%. Yield 71.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com