Lipid-lowering drug ezetimibe compound
The technology of a hypolipidemic drug and ezetimibe, which is applied in the field of medicine, can solve the problems of difficult patient acceptance, large preparation volume, and easy aging of solid dispersions after long-term storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of ezetimibe compound
[0033] (1) Grind the crude ezetimibe, pass it through a 60-mesh sieve, and then add it to a mixed solution of methylnitrile, ethanol, and acetone whose volume is 8 times the weight of ezetimibe. The volume ratio is 3:3:1, 65 rev / min stirring for 20 minutes;
[0034] (2) Add acetonitrile with a volume 5 times the weight of ezetimibe under stirring at 110 rpm, and raise the temperature to 30°C at the same time;
[0035] (3) After adding the solution, let it stand for 2 hours, and add deionized water at 0°C dropwise under the condition of stirring at 80 rpm. The volume of deionized water is 10 times the weight of ezetimibe, and drop it at a constant speed within 2 hours complete;
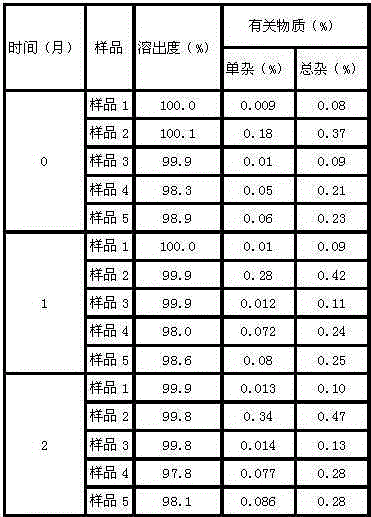
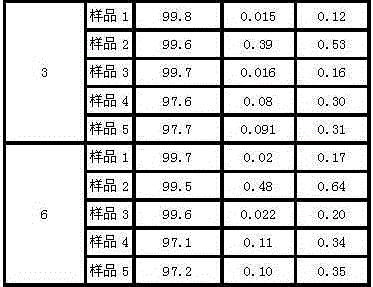
[0036] (4) After the dropwise addition was completed, the temperature was lowered to -10°C, and stirring was continued at a stirring rate of 40 rpm for 2 h, and the crystals were precipitated after standing for 1 h, filtered, and vacuum-dried to...
Embodiment 2
[0038] Example 2: Preparation of ezetimibe compound
[0039] (1) Grind the crude product of ezetimibe, pass it through a 75-mesh sieve, and then add it to a mixed solution of methylnitrile, ethanol, and acetone whose volume is 9 times the weight of ezetimibe. The volume ratio is 3:3:1, stirred at 70 rpm for 20 minutes;
[0040] (2) Add acetonitrile with a volume 6 times the weight of ezetimibe under stirring at 115 rpm, and raise the temperature to 32.5°C at the same time;
[0041] (3) After adding the solution, let it stand still for 2.5 hours, and add deionized water at 2.5°C dropwise under the condition of stirring at 85 rpm. added;
[0042] (4) After the dropwise addition was completed, the temperature was lowered to -7.5°C, and stirring was continued for 2.5 hours at a stirring rate of 45 rpm, and crystals were precipitated after standing for 1.5 hours, filtered, and vacuum-dried to obtain ezetimibe crystals.
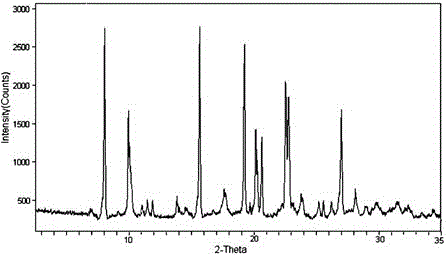
[0043] The X-ray powder diffraction spectrum obtained by...
Embodiment 3
[0044] Example 3: Preparation of ezetimibe compound
[0045] (1) Grind the crude ezetimibe, pass it through a 90-mesh sieve, and then add it to a mixed solution of methylnitrile, ethanol, and acetone whose volume is 10 times the weight of ezetimibe. The volume ratio is 3:3:1, stirred at 75 rpm for 20 minutes;
[0046] (2) Add acetonitrile with a volume 7 times the weight of ezetimibe under stirring at 120 rpm, and raise the temperature to 35°C at the same time;
[0047] (3) After adding the solution, let it stand still for 3 hours, and add deionized water at 5°C dropwise under the condition of stirring at 90 rpm. complete;
[0048] (4) After the dropwise addition was completed, the temperature was lowered to -5°C, and the stirring was continued for 3 hours at a stirring rate of 50 rpm, and the crystals were precipitated after standing for 2 hours, filtered, and vacuum-dried to obtain ezetimibe crystals.
[0049] The X-ray powder diffraction spectrum obtained by measuring th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com