Application of oleanolic acid type saponin compound in preparation of weight-losing and lipid-lowering drugs
A technology of oleanolic acid and saponins, which is applied in the field of pharmaceuticals or health products, and can solve the problems of low safety, many adverse reactions, and limited curative effect of weight-loss drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The instrument used in the present invention is a Bruker AV 600 nuclear magnetic resonance spectrometer (TMS is an internal standard), Buchi medium pressure preparation liquid phase, Agilent preparation chromatograph SD-1 (Agilent Technologies Inc.), and the detection wavelength λ is 215nm.
[0029] D101 macroporous resin, column chromatography silica gel, thin layer chromatography silica gel, acetonitrile and methanol are all commercially available. The medicinal materials used in the experiment were procured from the medicinal materials market.
[0030] Preparation of Compound 1-5
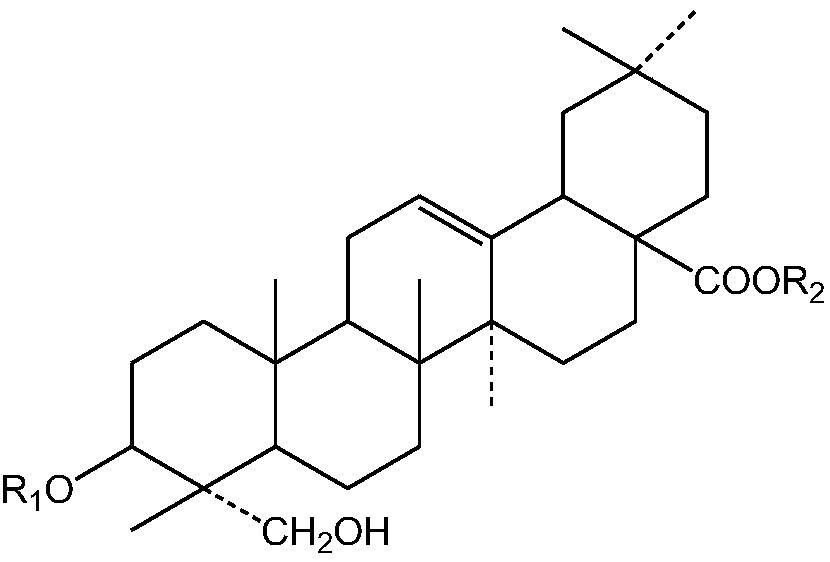
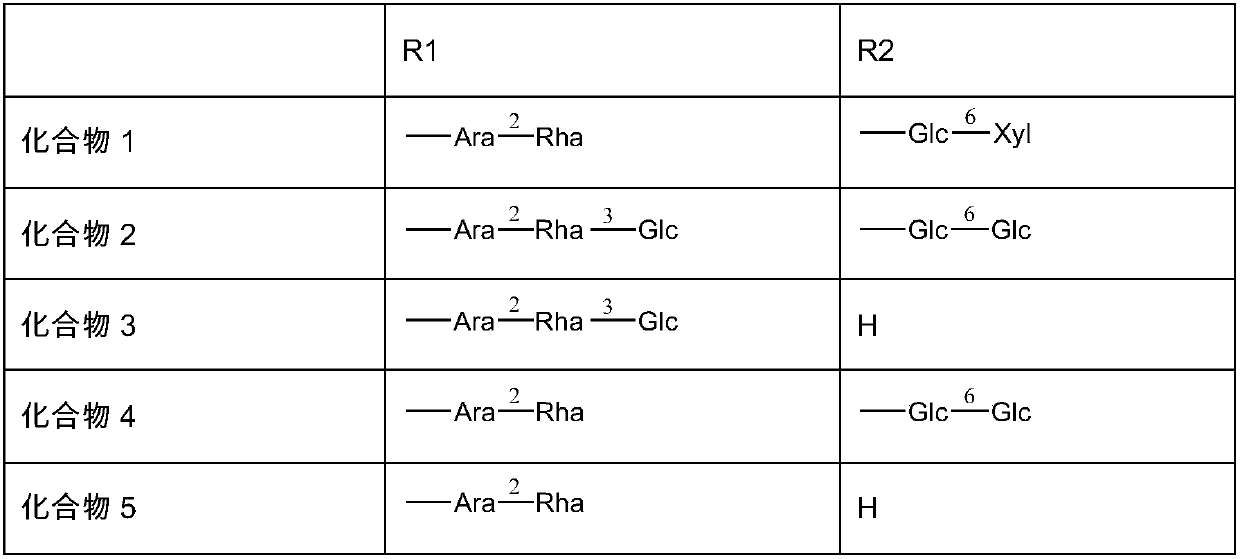
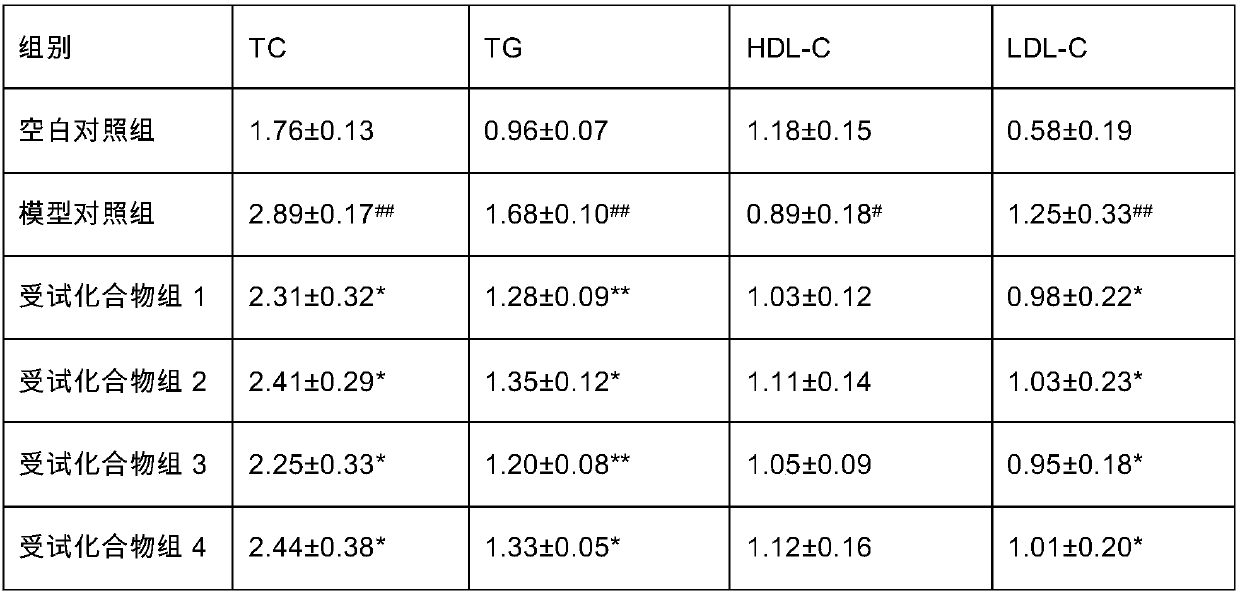
[0031]50kg of thorny dragon bud medicinal material, crushed to a coarse powder, and then refluxed with 70% ethanol to extract 3 times, each time for 2 hours, concentrated under reduced pressure to recover the solvent, and the obtained extract was extracted with ethyl acetate and n-butanol successively to obtain n-butanol Extract 1.2kg. The n-butanol part is separated by 50L D101 column c...
Embodiment 2
[0032] The preparation of embodiment 2 test composition 6-9
[0033] Test composition 6 (60% compound 1+40% fructooligosaccharide)
[0034] Accurately weigh 3.0 g of compound 1 obtained in Example 1, mix with 2.0 g of fructooligosaccharide, grind, stir evenly, and set aside.
[0035] Test composition 7 (80% compound 1+20% fructooligosaccharides)
[0036] Accurately weigh 4.0 g of Compound 1 prepared in Example 1, mix it with 1.0 g of fructo-oligosaccharide, grind it, stir it evenly, and set it aside.
[0037] Test composition 8 (60% compound 3+40% fructooligosaccharide)
[0038] Accurately weigh 3.0 g of Compound 3 prepared in Example 1, mix with 2.0 g of fructooligosaccharide, grind, stir evenly, and set aside.
[0039] Test composition 9 (80% compound 3+20% fructooligosaccharides)
[0040] Accurately weigh 4.0 g of Compound 3 prepared in Example 1, mix it with 1.0 g of fructooligosaccharide, grind, stir evenly, and set aside.
Embodiment 3
[0041] Embodiment 3 preparation of compound tablet of the present invention
[0042] Take compound 1 or its pharmaceutically acceptable salt or ester (3g), lactose (4g), hydroxypropyl cellulose (3g), starch (2g) and mix them with a mixer, pass through a 60 mesh sieve, add micronized silica gel (1g) , magnesium stearate (0.5g) are mixed homogeneously, and the gained mixed product is compressed into tablets with a tablet machine to obtain tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com