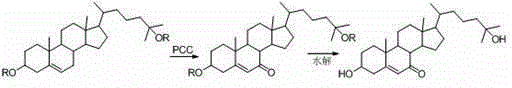
Synthesis method for 25-hydroxy-7-ketocholesterol
A technology of hydroxycholesterol and its synthesis method, which is applied in the fields of steroids and organic chemistry, can solve the problems of difficult industrialization, troublesome post-processing, and great environmental protection hazards, and achieve the effect of less three wastes, low cost, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] At room temperature (20-25°C), dissolve 4.86 g (10 mmol) of 25-hydroxycholesterol diacetate in 50 mL of butanone, add 1.63 g (10 mmol) of N-hydroxyphthalimide, 48 mg of benzoyl peroxide, stirred evenly, continued to feed air, reacted for 8 hours, TLC traced to the complete reaction of 25-hydroxycholesterol diacetate, cooled down to 0°C, and precipitated catalyst N-hydroxyphthaloyl Amine, filter and recover N-hydroxyphthalimide, add 0.49g dehydrating agent Pt / C to the filtrate, stir and react at 0°C for 10 hours, after the reaction is complete, filter and recover Pt / C, add sodium hydroxide Aqueous solution, pH ≥ 13, hydrolyzed at 50°C for 1 hour, separated to remove the water phase, and concentrated the organic phase to obtain a light yellow solid. The crude product was recrystallized with 20 mL of methanol to obtain 3.62 g of 25-hydroxy-7-ketocholesterol. 87% yield, white solid, melting point 178~180℃, 1 HNMR (CDCl 3 , 400MHz): δ 5.67 (d, 1H, 6-CH), 3.66 (m, 1H, 3-CH)...
Embodiment 2
[0027] At room temperature (20-25°C), dissolve 4.86 g (10 mmol) of 25-hydroxycholesterol diacetate in 50 mL of butanone, add 1.63 g (10 mmol) of N-hydroxyphthalimide, 48 mg of benzoyl peroxide, stirred evenly and continued to pass in the air, reacted for 8 hours, TLC traced to the complete reaction of 25-hydroxycholesterol diacetate, cooled down to 0°C, and the catalyst N-hydroxyphthaloyl was precipitated Imine, filtered, recovered N-hydroxyphthalimide, added 2.02g (20 mmol) triethylamine and 3.81g (20 mmol) p-toluenesulfonyl chloride to the filtrate, stirred and reacted at 0°C for 8 hours, After the reaction is complete, filter to remove salt, add 20% aqueous sodium hydroxide solution, control pH ≥ 13, hydrolyze at 50°C for 1 hour, remove the aqueous phase by layering, and concentrate the organic phase to obtain a light yellow solid. The crude product is recrystallized with 20 mL of methanol 3.41 g of 25-hydroxy-7-ketocholesterol was obtained as a white solid with a yield of ...
Embodiment 3
[0029] At room temperature (20-25°C), dissolve 4.86 g (10 mmol) of 25-hydroxycholesterol diacetate in 50 mL of acetone, add 1.15 g (10 mmol) of N-hydroxysuccinimide, and 48 mg Oxidize benzoyl, stir evenly and continue to feed air, react for 12 hours, after TLC tracking the reaction is complete, cool down to 0°C, precipitate the catalyst, filter, recover N-hydroxysuccinimide, add Pt / C to the filtrate 0.49g, stirred and reacted at 0°C for 10 hours. After the reaction is complete, filter and recover Pt / C, add aqueous sodium hydroxide solution, control pH ≥ 13, hydrolyze at 50°C for 1 hour, remove the aqueous phase by layering, and concentrate the organic phase to obtain a light yellow solid. The crude product is recrystallized with 20 mL of methanol 3.37 g of 25-hydroxy-7-ketocholesterol was obtained, with a yield of 81%, as a white solid with a melting point of 177-179°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com