Synthetic method of N, N-diaryl-2-bromine-6-naphthylamine and application thereof
A synthesis method, diaryl technology, applied in the application of organic semiconductor materials, the field of synthesis of N,N-diaryl-2-bromo-6-naphthylamine, can solve the difficulty of aromatic amine synthesis, comprehensive production Problems such as low rate and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
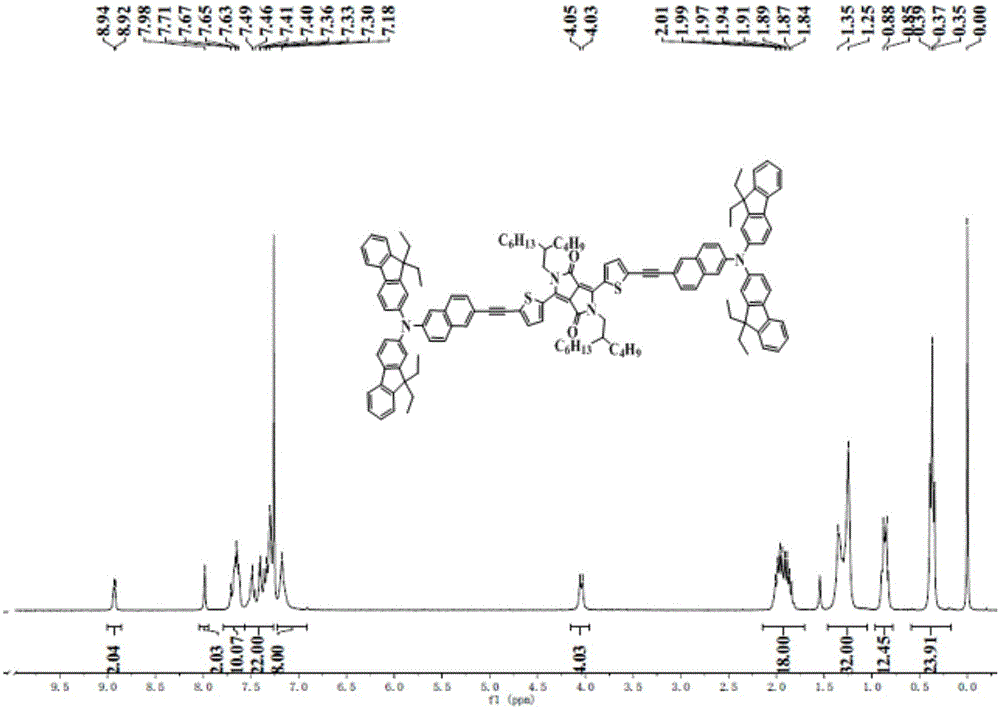
[0080] The N,N-diaryl-2-bromo-6-naphthylamine (DT-DPP(FNN-A) of this embodiment 2) synthetic method, comprising the following steps:
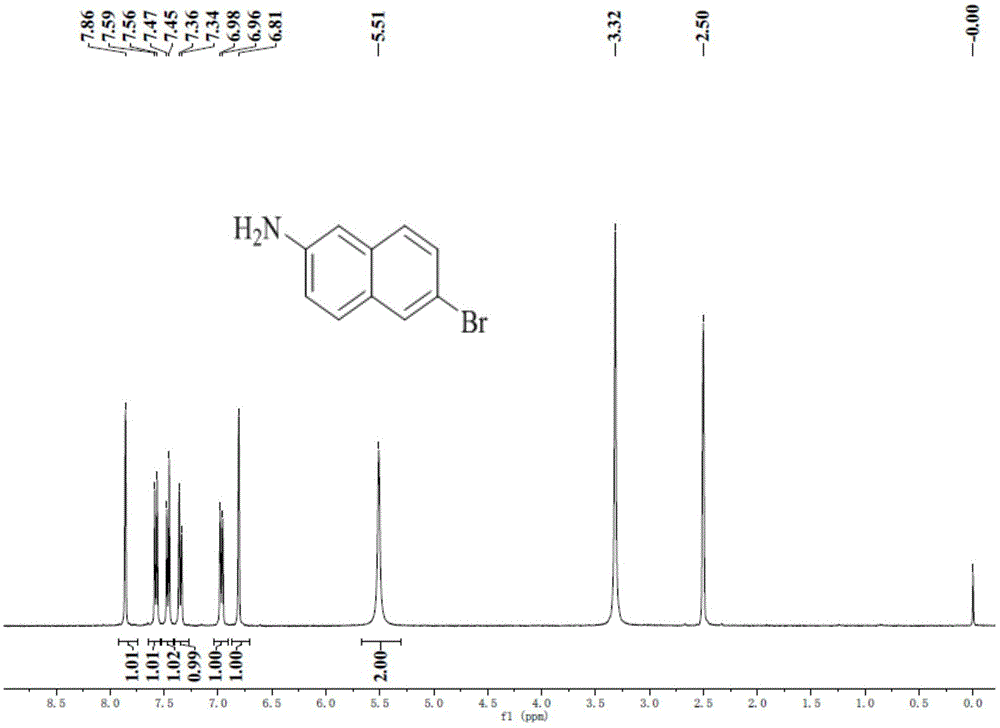
[0081] Synthesis of step one compound 6-bromo-2-naphthylamine (1)
[0082] After dissolving tunic acid (15g, 67.2mmol) in glacial acetic acid at 70°C, liquid bromine (21.47g, 134mmol) was added dropwise and reacted at constant temperature for 2h. Then the temperature was lowered to 60°C, tin powder (8g, 67.4mmol) and concentrated hydrochloric acid (95ml) were added carefully, and the reaction was refluxed for 3h. After the reaction, the glacial acetic acid was distilled off under reduced pressure, and the residue was neutralized with NaOH solution until the solution was weakly alkaline, and extracted with dichloromethane. The organic phase was dried and filtered over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The obtained crude product was purified by silica gel column chromatography and recrystall...
Embodiment 2
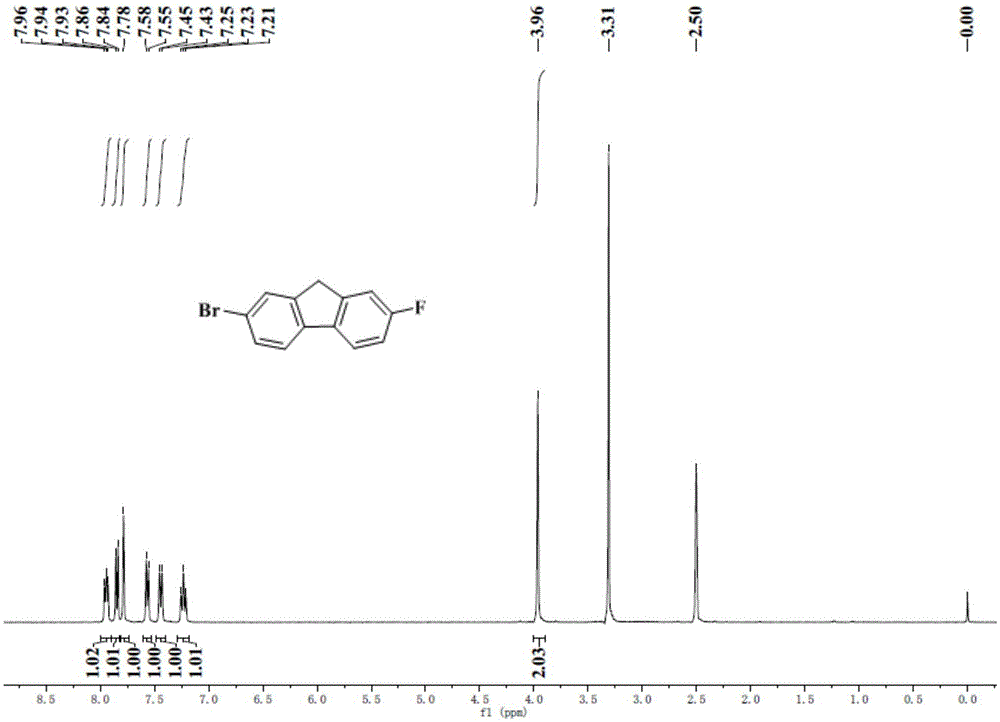
[0104] The N,N-diaryl-2-bromo-6-naphthylamine (DT-DPP(FFNN-A) of this embodiment 2 ) synthetic method, using 2-bromofluorene as a raw material to synthesize 2-fluoro-7-bromofluorene through a new synthetic route, and using this unit to synthesize a new type of organic photoelectric material DT-DPP (FFNN-A) with a route similar to Example 1 2 ,Specific steps are as follows:
[0105] Synthesis of step 1 compound 2-nitro-7-bromofluorene (1)
[0106] 2-Bromofluorene (7g, 28.6mmol) was dissolved in 40mL of glacial acetic acid, heated to 60°C, and 20mL of concentrated nitric acid / glacial acetic acid (3:1, v / v) was added dropwise under vigorous stirring. After constant temperature reaction for 1.5 h, it was cooled to room temperature and extracted with dichloromethane / water. The organic phase was dried and filtered with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The obtained crude product was purified by recrystallization to obtain 7.4 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com