The preparation method of 1,3,6-hexanetrinitrile
A technology of hexanetrinitrile and hexene, which is applied in the field of compound preparation, can solve the problems of poor whiteness and low purity, and achieve the effects of high yield, high product purity and simple reaction control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
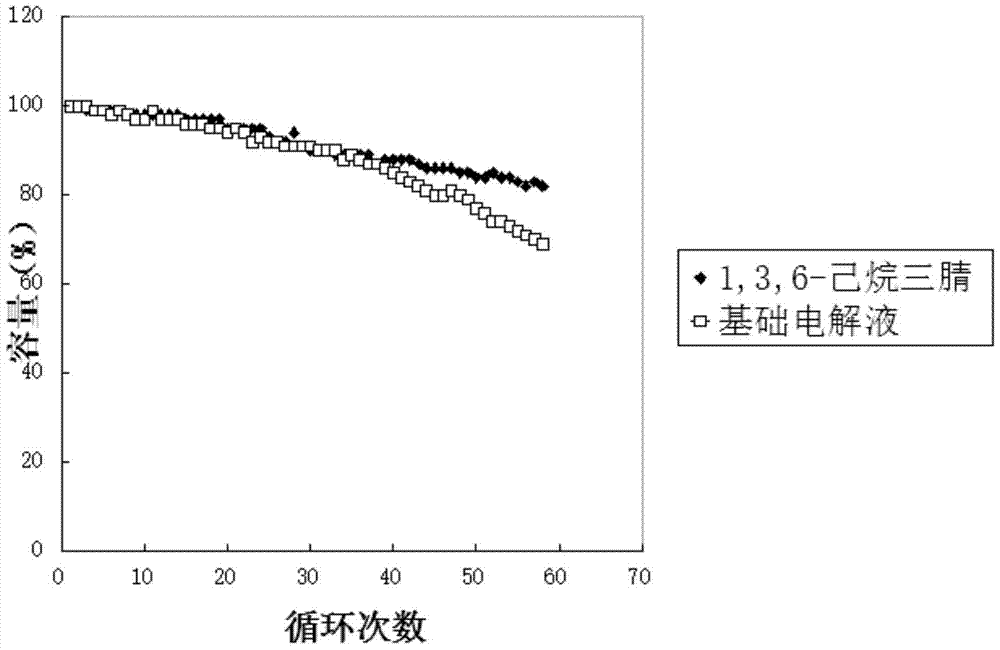
Image
Examples
Embodiment 1
[0020] The preparation method of 1,3,6-hexanetrinitrile comprises the following steps:
[0021] A. Preparation of 1,6-dicyano-2-hexene: With 1,4-dioxane as the reaction solvent, 1mol 1,6-dichloro-2-hexene, 0.5g CuCl and 2mol NaCN Place in 1,4-dioxane, and carry out substitution reaction at 40°C for 2 hours to obtain 1,6-dicyano-2-hexene;
[0022] B. Preparation of 1,3,6-hexanetrinitrile: react the 1,6-dicyano-2-hexene prepared in step A with 90g HCl methanol solution at 40°C for 4h, then add NaCN to it at 40°C The substitution reaction was carried out at °C for 2 hours to obtain 1,3,6-hexanetrinitrile with a calculated yield of 52.1%. The purity of the product was 99.55%, the product color number APHA was 15, and the product moisture content was 16PPM.
Embodiment 2
[0024] The preparation method of 1,3,6-hexanetrinitrile comprises the following steps:
[0025] Preparation of A, 1,6-dicyano-2-hexene: 1mol 1,6-dichloro-2-hexene, 0.5g Cu 2 O and 2.5mol NaCN were placed in 1,4-dioxane, and the substitution reaction was carried out at 80°C for 5 hours to obtain 1,6-dicyano-2-hexene;
[0026] B. Preparation of 1,3,6-hexanetrinitrile: react 1,6-dicyano-2-hexene prepared in step A with 90 g HCl methanol solution at 50°C for 5 hours, then add NaCN to it at 80 The substitution reaction was carried out at °C for 5 hours to obtain 1,3,6-hexanetrinitrile with a calculated yield of 61.2%. The purity of the product was 99.63%, the product color APHA was 13, and the product moisture was 18PPM.
Embodiment 3
[0028] The preparation method of 1,3,6-hexanetrinitrile comprises the following steps:
[0029] A. Preparation of 1,6-dicyano-2-hexene: With 1,4-dioxane as the reaction solvent, 1mol 1,6-dichloro-2-hexene, 0.5g CuBr and 2.3mol NaCN Place in 1,4-dioxane, and carry out substitution reaction at 101°C for 7 hours to obtain 1,6-dicyano-2-hexene;
[0030] B. Preparation of 1,3,6-hexanetrinitrile: react the 1,6-dicyano-2-hexene prepared in step A with 90g HCl methanol solution at 60°C for 6h, then add NaCN to it at 101 The substitution reaction was carried out at °C for 7 hours to obtain 1,3,6-hexanetrinitrile with a calculated yield of 57.5%. The purity of the product was 99.54%, the product color APHA was 17, and the product moisture was 16PPM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com