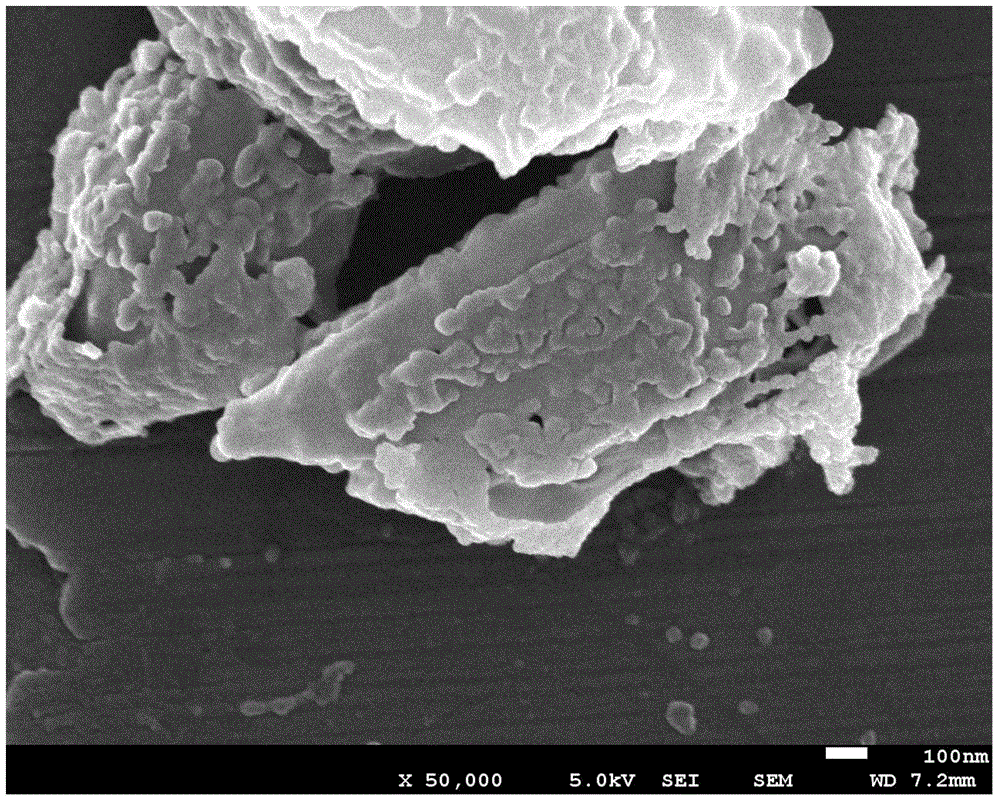
Preparation method of silicon-dioxide-coated sulfur microcapsules
A technology of sulfur and coating, applied in the field of preparation of coated sulfur, can solve the problems of restricting the development of insoluble sulfur, poor high temperature stability, poor dispersibility, etc., and achieves good monodispersity, small particle size and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
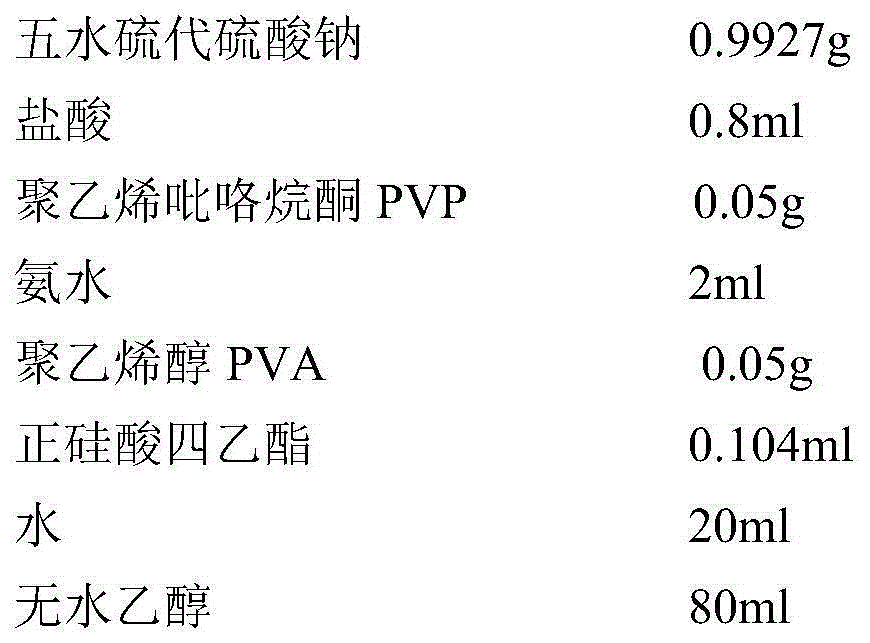
[0016] The composition and consumption of raw materials for preparing coated sulfur are as follows:
[0017]
[0018] The preparation method of silica-coated sulfur is as follows:
[0019] (1) Add 0.9927g of sodium thiosulfate pentahydrate to an aqueous solution containing 0.05g of PVA at room temperature, and after it dissolves, add 0.8ml of dilute hydrochloric acid dropwise, then stir and react at room temperature for 2 hours to prepare a sulfur water dispersion ;
[0020] (2) Centrifuge and wash the above-mentioned sulfur water dispersion twice, then ultrasonically disperse it in 20ml aqueous solution containing 0.05gPVP, then add 80ml of absolute ethanol, and continue ultrasonically dispersing into a homogeneous emulsion;
[0021] (3) Add dropwise 50ml ethanol solution containing 0.104g tetraethyl orthosilicate upwards as in the sulfur dispersion with a constant pressure dropping funnel, and stir at room temperature for 2 hours after the dropwise addition;
[0022] (4...
Embodiment 2
[0025] The composition and consumption of raw materials for preparing coated sulfur are as follows:
[0026]
[0027] The preparation method of silica-coated sulfur is as follows:
[0028] (1) Add 0.9927g of sodium thiosulfate pentahydrate to an aqueous solution containing 0.2g of PVA at room temperature, and after it dissolves, add 0.8ml of dilute hydrochloric acid dropwise, then stir and react at room temperature for 2 hours to prepare a sulfur water dispersion ;
[0029] (2) Centrifuge and wash the above-mentioned sulfur water dispersion twice, then ultrasonically disperse it in 20ml aqueous solution containing 0.05gPVP, then add 80ml of absolute ethanol, and continue ultrasonically dispersing into a homogeneous emulsion;
[0030] (3) Add dropwise 50ml ethanol solution containing 0.208g tetraethyl orthosilicate upwards as in the sulfur dispersion with a constant pressure dropping funnel, and stir at room temperature for 2 hours after the dropwise addition;
[0031] (4)...
Embodiment 3
[0034] The composition and consumption of raw materials for preparing coated sulfur are as follows:
[0035]
[0036] The preparation method of silica-coated sulfur is as follows:
[0037] (1) After preparing an aqueous solution containing 0.05g of PVP at room temperature, add 4.9636g of sodium thiosulfate pentahydrate to dissolve, then add 4ml of dilute hydrochloric acid dropwise, and then stir and react at room temperature for 2 hours to prepare a sulfur water dispersion;
[0038] (2) Centrifuge and wash the above-mentioned sulfur water dispersion twice, then ultrasonically disperse it in 20ml aqueous solution containing 0.1g gelatin, then add 80ml of absolute ethanol, and continue to ultrasonically disperse into a homogeneous emulsion;
[0039] (3) Use a constant pressure dropping funnel to add dropwise 20ml of ethanol solution containing 1ml tetraethyl orthosilicate to the sulfur dispersion, and stir at room temperature for 2 hours after the dropwise addition;
[0040]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com