Catalyst for the production of polyols having lower amounts of high molecular weight tail
A catalyst, weight technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, catalytic reaction, etc., can solve the problem of reducing and not disclosing the amount of high molecular weight tailings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
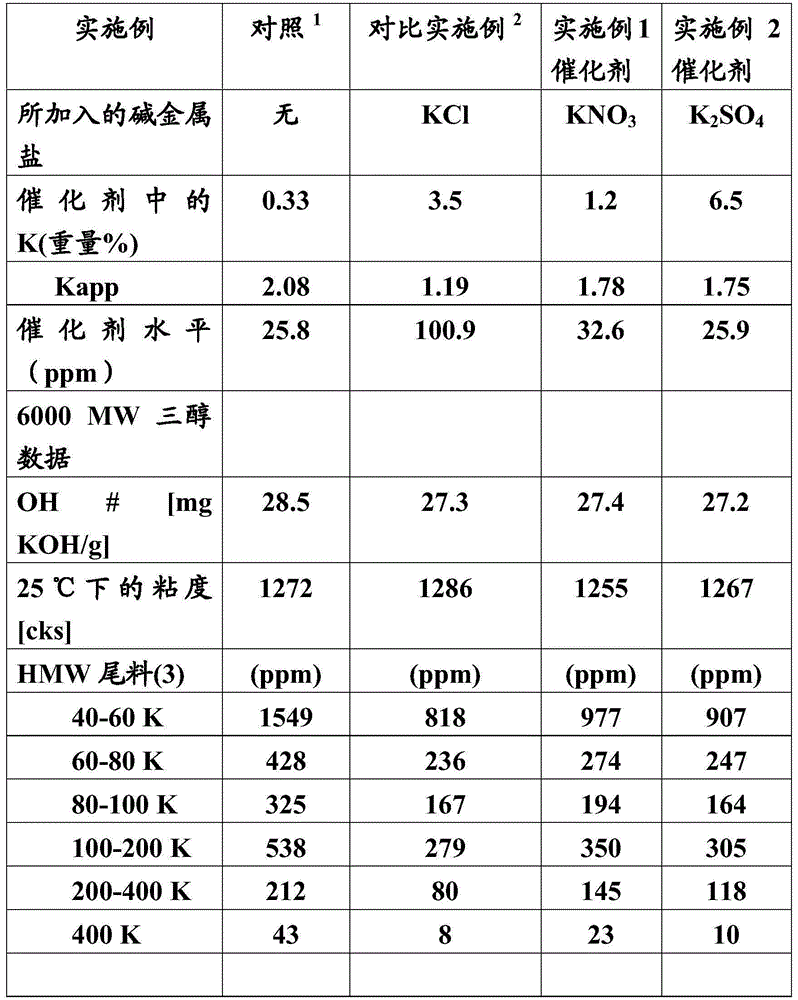
Examples
Embodiment 1
[0055] Preparation of DMC catalyst using potassium nitrate and polyoxypropylene glycol:
[0056] 120 g (0.88 mol) of ZnCl in 230 g of deionized water were prepared in a one-liter stirred reactor 2 and 38 g (0.513 mol) of tert-butanol in water and heated to 50° C. (solution 1). Potassium hexacyanocobaltate (8.1 g, 0.0243 mol) and potassium nitrate (5.85 g, 0.0579 mol) were dissolved in deionized water (110 g) and tert-butanol (17.2 g, 0.232 mol) in a 500 ml beaker (Solution 2 ). Solution 3 was prepared by dissolving 1000 molecular weight (mol.wt.) polyoxypropylene diol (8 grams) in deionized water (50 grams) and t-butanol (2 grams). Solution 2 was added to Solution 1 at 3.0 g / min over 42 minutes while mixing at 500 rpm. The reaction temperature was maintained at 50°C during the reaction by using an internal coil for heating or cooling. After addition, mixing was continued for 20 minutes at 500 rpm. The mixing speed was reduced and solution 3 was added, after which slow sti...
Embodiment 2
[0060] Preparation of DMC catalyst using potassium sulfate and polyoxypropylene glycol:
[0061] A solution containing 120 g (0.88 mol) of ZnCl was prepared in a one-liter stirred reactor 2 , deionized water (230 g), tert-butanol (38 g, 0.513 mol) and potassium sulfate (5.03 g, 0.029 mol) in water and heated to 50° C. (Solution 1). Potassium hexacyanocobaltate (8.1 grams, 0.0243 moles) was dissolved in deionized water (110 grams) and tert-butanol (17.2 grams, 0.232 moles) in a 500 ml beaker (Solution 2). Solution 3 was prepared by dissolving 1000 molecular weight polyoxypropylene diol (8 grams) in deionized water (50 grams) and t-butanol (2 grams). Solution 2 was added to Solution 1 at 3.0 g / min over 42 minutes while mixing at 500 rpm. The reaction temperature was maintained at 50°C during the reaction by using internal coils for heating or cooling. After addition, mixing was continued for 20 minutes at 500 rpm. The mixing speed was reduced and solution 3 was added, after ...
Embodiment 3
[0065] Preparation of DMC catalyst using potassium sulfate and 2000MW polyoxypropylene diol:
[0066] An aqueous solution containing 120 g (0.88 mol) of zinc chloride, 38 g (0.513 mol) of tert-butanol, and 5.8 g (0.033 mol) of potassium sulfate in 230 g of deionized water was prepared in a one-liter stirred reactor and heated to 50°C (Solution 1). Potassium hexacyanocobaltate (8.91 grams, 0.030 moles) was dissolved in deionized water (110 grams) and tert-butanol (18.92 grams, 0.255 moles) in a 500 ml beaker (Solution 2). Solution 3 was prepared by dissolving 2000 molecular weight polyoxypropylene diol (8 grams) in deionized water (50 grams) and t-butanol (2 grams). Solution 2 was added to Solution 1 at 3.0 g / min over 42 minutes while mixing at 500 rpm. The reaction temperature was maintained at 50°C during the reaction by using internal coils for heating or cooling. After addition, mixing was continued for 20 minutes at 500 rpm. The mixing speed was reduced and solution 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com